|
Strukturbericht Designation
In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as supplement to space group crystal structures designations, especially historically. Each Strukturbericht designation is described by a single space group, but the designation includes additional information about the positions of the individual atoms, rather than just the symmetry of the crystal structure. While Strukturbericht symbols exist for many of the earliest observed and most common crystal structures, the system is not comprehensive, and is no longer being updated. Modern databases such as Inorganic Crystal Structure Database index thousands of structure types directly by the prototype compound (i.e. "the NaCl structure" instead of "the B1 structure"). These are essentially equivalent to the old Stukturbericht designations. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
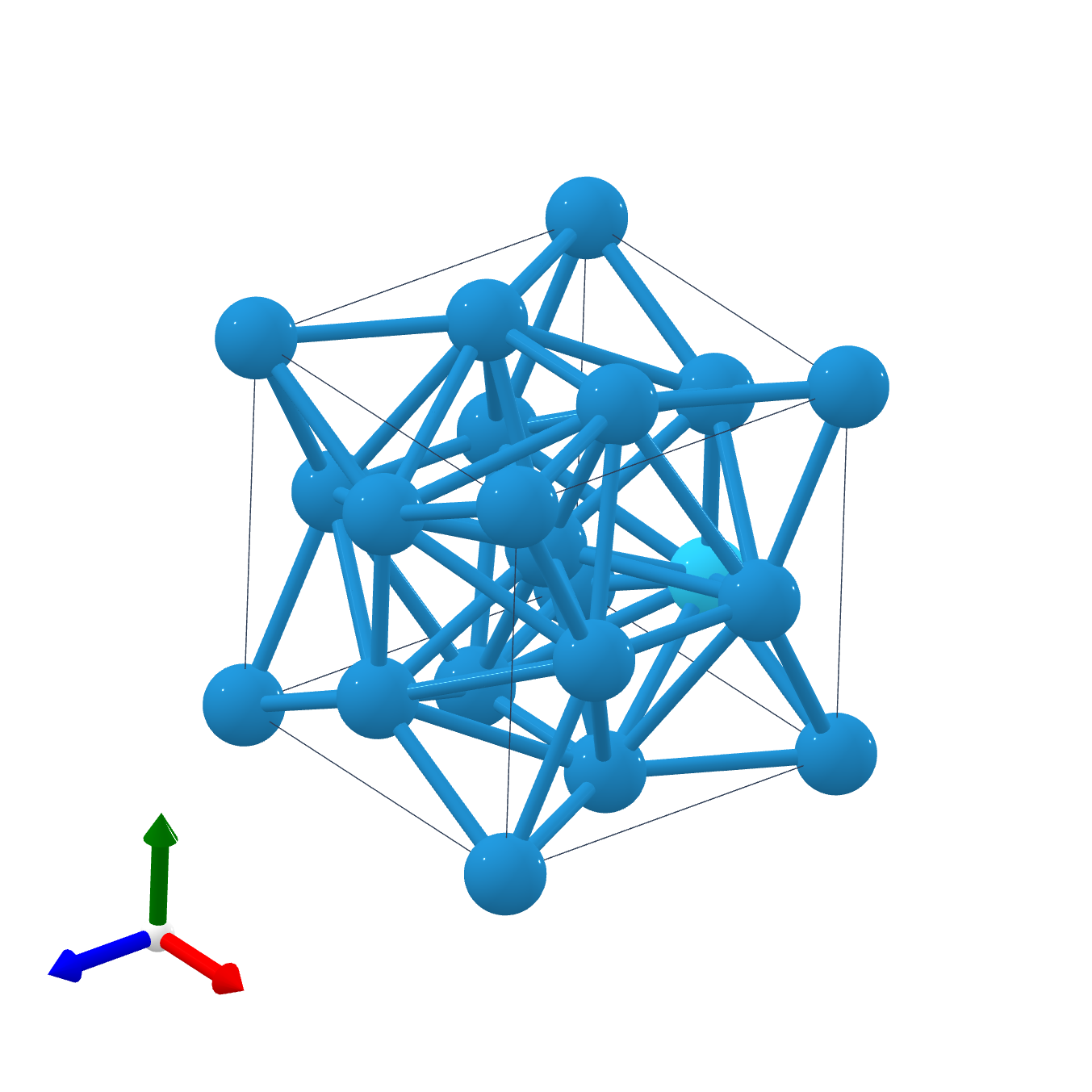
Beta-Tungsten
Beta-tungsten (β-W) is a metastable phase of tungsten widely observed in tungsten thin films. While the commonly existing stable α-W has a body-centered cubic ( A2) structure, β-W adopts the topologically close-packed A15 structure containing eight atoms per unit cell, and it irreversibly transforms to the stable α phase through thermal annealing of up to 650°C. It has been found that β-W possesses the giant spin Hall effect, wherein the applied charge current generates a transverse spin current, and this leads to potential applications in magnetoresistive random access memory devices. History β-W was first observed by Hartmann et al. in 1931 as part of the dendritic metallic deposit formed on the cathode after electrolysis of phosphate melts below 650°C. In the beginning stages of research into β-W, oxygen was commonly found to promote the formation of the β-W structure, thus discussions of whether the β-W structure is a phase of single-element tungsten or a tun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Uc
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year. Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coatings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A15 Phases
The A15 phases (also known as β-W or Cr3Si structure types) are series of intermetallic compounds with the chemical formula ''A''3''B'' (where A is a transition metal and B can be any element) and a specific structure. The A15 phase is also one of the members in the Frank–Kasper phases family. Many of these compounds have superconductivity at around , which is comparatively high, and remain superconductive in magnetic fields of tens of teslas (hundreds of kilogauss). This kind of superconductivity ( Type-II superconductivity) is an important area of study as it has several practical applications. History The first time that A15 structure was observed was in 1931 when an electrolytically deposited layer of tungsten was examined. Discussion of whether the β-tungsten structure is an allotrope of tungsten or the structure of a tungsten suboxide was long-standing, but since the 1950s there has been many publications showing that the material is a true allotrope of tungsten. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese was first isolated in 1774. It is familiar in the laborator ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the Symbol (chemistry), symbol Ga and atomic number 31. Discovered by France, French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in boron group, group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in Standard conditions for temperature and pressure, standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidal fracture, conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
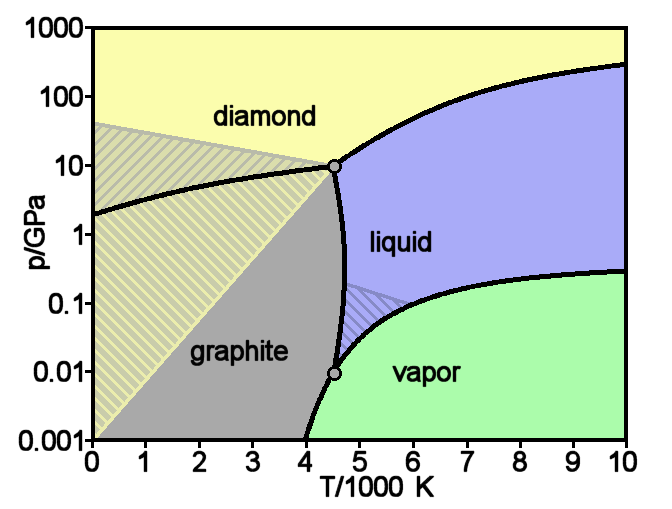
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |