|
Sommerfeld Expansion
A Sommerfeld expansion is an approximation method developed by Arnold Sommerfeld for a certain class of integrals which are common in condensed matter and statistical physics. Physically, the integrals represent statistical averages using the Fermi–Dirac distribution. When the inverse temperature \beta is a large quantity, the integral can be expanded in terms of \beta as :\int_^\infty \frac\,\mathrm\varepsilon = \int_^\mu H(\varepsilon)\,\mathrm\varepsilon + \frac\left(\frac\right)^2H^\prime(\mu) + O \left(\frac\right)^4 where H^\prime(\mu) is used to denote the derivative of H(\varepsilon) evaluated at \varepsilon = \mu and where the O(x^n) notation refers to limiting behavior of order x^n. The expansion is only valid if H(\varepsilon) vanishes as \varepsilon \rightarrow -\infty and goes no faster than polynomially in \varepsilon as \varepsilon \rightarrow \infty. If the integral is from zero to infinity, then the integral in the first term of the expansion is from zero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld, (; 5 December 1868 – 26 April 1951) was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and mentored many students for the new era of theoretical physics. He served as doctoral supervisor for many Nobel Prize winners in physics and chemistry (only J. J. Thomson's record of mentorship is comparable to his). He introduced the second quantum number ( azimuthal quantum number) and the third quantum number ( magnetic quantum number). He also introduced the fine-structure constant and pioneered X-ray wave theory. Early life and education Sommerfeld was born in 1868 to a family with deep ancestral roots in Prussia. His mother Cäcilie Matthias (1839–1902) was the daughter of a Potsdam builder. His father Franz Sommerfeld (1820–1906) was a physician from a leading family in Königsberg, where Arnold's grandfather had resettled from the hinterland in 1822 for a career as Court Pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integral
In mathematics, an integral assigns numbers to functions in a way that describes displacement, area, volume, and other concepts that arise by combining infinitesimal data. The process of finding integrals is called integration. Along with differentiation, integration is a fundamental, essential operation of calculus,Integral calculus is a very well established mathematical discipline for which there are many sources. See and , for example. and serves as a tool to solve problems in mathematics and physics involving the area of an arbitrary shape, the length of a curve, and the volume of a solid, among others. The integrals enumerated here are those termed definite integrals, which can be interpreted as the signed area of the region in the plane that is bounded by the graph of a given function between two points in the real line. Conventionally, areas above the horizontal axis of the plane are positive while areas below are negative. Integrals also refer to the concept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensed-matter Physics
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases which arise from electromagnetic forces between atoms. More generally, the subject deals with "condensed" phases of matter: systems of many constituents with strong interactions between them. More exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, and the Bose–Einstein condensate found in ultracold atomic systems. Condensed matter physicists seek to understand the behavior of these phases by experiments to measure various material properties, and by applying the physical laws of quantum mechanics, electromagnetism, statistical mechanics, and other theories to develop mathematical models. The diversity of systems and phenomena available for study makes condensed matter ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Physics
Statistical physics is a branch of physics that evolved from a foundation of statistical mechanics, which uses methods of probability theory and statistics, and particularly the mathematical tools for dealing with large populations and approximations, in solving physical problems. It can describe a wide variety of fields with an inherently stochastic nature. Its applications include many problems in the fields of physics, biology, chemistry, and neuroscience. Its main purpose is to clarify the properties of matter in aggregate, in terms of physical laws governing atomic motion. Statistical mechanics develop the phenomenological results of thermodynamics from a probabilistic examination of the underlying microscopic systems. Historically, one of the first topics in physics where statistical methods were applied was the field of classical mechanics, which is concerned with the motion of particles or objects when subjected to a force. Scope Statistical physics explains and quanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi–Dirac Statistics
Fermi–Dirac statistics (F–D statistics) is a type of quantum statistics that applies to the physics of a system consisting of many non-interacting, identical particles that obey the Pauli exclusion principle. A result is the Fermi–Dirac distribution of particles over energy states. It is named after Enrico Fermi and Paul Dirac, each of whom derived the distribution independently in 1926 (although Fermi derived it before Dirac). Fermi–Dirac statistics is a part of the field of statistical mechanics and uses the principles of quantum mechanics. F–D statistics applies to identical and indistinguishable particles with half-integer spin (1/2, 3/2, etc.), called fermions, in thermodynamic equilibrium. For the case of negligible interaction between particles, the system can be described in terms of single-particle energy states. A result is the F–D distribution of particles over these states where no two particles can occupy the same state, which has a considerable ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Beta
In statistical thermodynamics, thermodynamic beta, also known as coldness, is the reciprocal of the thermodynamic temperature of a system:\beta = \frac (where is the temperature and is Boltzmann constant).J. Meixner (1975) "Coldness and Temperature", ''Archive for Rational Mechanics and Analysis'' 57:3, 281-29abstract It was originally introduced in 1971 (as "coldness function") by , one of the proponents of the rational thermodynamics school of thought, based on earlier proposals for a "reciprocal temperature" function.Day, W.A. and Gurtin, Morton E. (1969) "On the symmetry of the conductivity tensor and other restrictions in the nonlinear theory of heat conduction", ''Archive for Rational Mechanics and Analysis'' 33:1, 26-32 (Springer-Verlag)abstractJ. Castle, W. Emmenish, R. Henkes, R. Miller, and J. Rayne (1965) Science by Degrees: ''Temperature from Zero to Zero'' (Westinghouse Search Book Series, Walker and Company, New York). Thermodynamic beta has units reciprocal to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Heat Capacity
In solid state physics the electronic specific heat, sometimes called the electron heat capacity, is the specific heat of an electron gas. Heat is transported by phonons and by free electrons in solids. For pure metals, however, the electronic contributions dominate in the thermal conductivity. In impure metals, the electron mean free path is reduced by collisions with impurities, and the phonon contribution may be comparable with the electronic contribution. Introduction Although the Drude model was fairly successful in describing the electron motion within metals, it has some erroneous aspects: it predicts the Hall coefficient with the wrong sign compared to experimental measurements, the assumed additional electronic heat capacity to the lattice heat capacity, namely \tfrac k_ per electron at elevated temperatures, is also inconsistent with experimental values, since measurements of metals show no deviation from the Dulong–Petit law. The observed electronic contribution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Electron Model
In solid-state physics, the free electron model is a quantum mechanical model for the behaviour of charge carriers in a metallic solid. It was developed in 1927, principally by Arnold Sommerfeld, who combined the classical Drude model with quantum mechanical Fermi–Dirac statistics and hence it is also known as the Drude–Sommerfeld model. Given its simplicity, it is surprisingly successful in explaining many experimental phenomena, especially * the Wiedemann–Franz law which relates electrical conductivity and thermal conductivity; * the temperature dependence of the electron heat capacity; * the shape of the electronic density of states; * the range of binding energy values; * electrical conductivities; * the Seebeck coefficient of the thermoelectric effect; * thermal electron emission and field electron emission from bulk metals. The free electron model solved many of the inconsistencies related to the Drude model and gave insight into several other properties of metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Beta
In statistical thermodynamics, thermodynamic beta, also known as coldness, is the reciprocal of the thermodynamic temperature of a system:\beta = \frac (where is the temperature and is Boltzmann constant).J. Meixner (1975) "Coldness and Temperature", ''Archive for Rational Mechanics and Analysis'' 57:3, 281-29abstract It was originally introduced in 1971 (as "coldness function") by , one of the proponents of the rational thermodynamics school of thought, based on earlier proposals for a "reciprocal temperature" function.Day, W.A. and Gurtin, Morton E. (1969) "On the symmetry of the conductivity tensor and other restrictions in the nonlinear theory of heat conduction", ''Archive for Rational Mechanics and Analysis'' 33:1, 26-32 (Springer-Verlag)abstractJ. Castle, W. Emmenish, R. Henkes, R. Miller, and J. Rayne (1965) Science by Degrees: ''Temperature from Zero to Zero'' (Westinghouse Search Book Series, Walker and Company, New York). Thermodynamic beta has units reciprocal to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
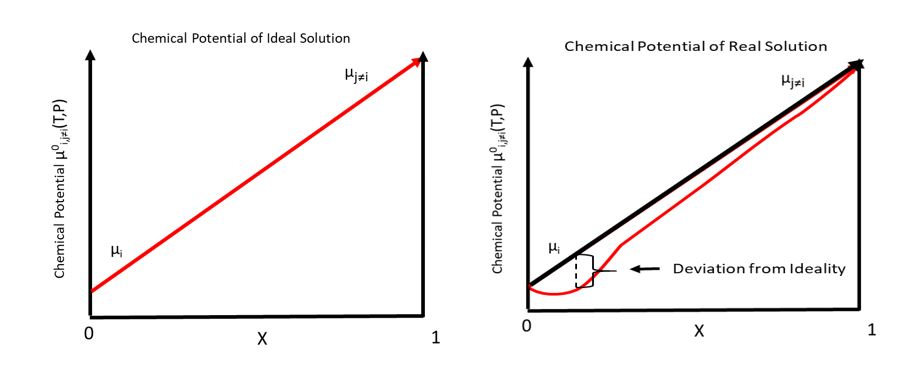
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer. Thermometers are calibrated in various Conversion of units of temperature, temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann's Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |