|
Silver Bromide
Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite. Preparation Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide: :AgNO3(aq) + KBr(aq) → AgBr(s)+ KNO3(aq) Although less convenient, the compound can also be prepared directly from its elements. Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated onto a film or other support. The crystals are formed by precipitation in a controlled environment to produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromargyrite
Bromyrite or bromargyrite is a natural mineral form of silver bromide found mainly in Mexico and Chile. Hardness is 1.5 to 2. Related are chlorargyrite and iodyrite. It was first described in 1859 for an occurrence in Plateros, Zacatecas, Mexico Mexico (Spanish language, Spanish: México), officially the United Mexican States, is a List of sovereign states, country in the southern portion of North America. It is borders of Mexico, bordered to the north by the United States; to the so ... where it occurred in a silver deposit as an oxidation product of primary ore minerals. It occurs in arid environments along with native silver, iodargyrite and smithsonite along with iron and manganese oxide minerals. References Bromides Silver minerals Halide minerals Cubic minerals Minerals in space group 225 {{Halide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lattice Face Centered Cubic
Lattice may refer to: Arts and design * Latticework, an ornamental criss-crossed framework, an arrangement of crossing laths or other thin strips of material * Lattice (music), an organized grid model of pitch ratios * Lattice (pastry), an ornamental pattern of crossing strips of pastry Companies * Lattice Engines, a technology company specializing in business applications for marketing and sales * Lattice Group, a former British gas transmission business * Lattice Semiconductor, a US-based integrated circuit manufacturer Science, technology, and mathematics Mathematics * Lattice (group), a repeating arrangement of points ** Lattice (discrete subgroup), a discrete subgroup of a topological group whose quotient carries an invariant finite Borel measure ** Lattice (module), a module over a ring which is embedded in a vector space over a field ** Lattice graph, a graph that can be drawn within a repeating arrangement of points ** Lattice-based cryptography, encryption syste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
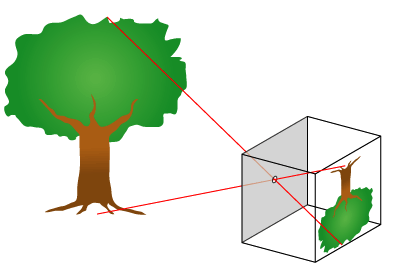
Science Of Photography
The science of photography is the use of chemistry and physics in all aspects of photography. This applies to the camera, its lenses, physical operation of the camera, electronic camera internals, and the process of developing film in order to take and develop pictures properly. Optics Camera obscura The fundamental technology of most photography, whether digital or analog, is the camera obscura effect and its ability to transform of a three dimensional scene into a two dimensional image. At its most basic, a camera obscura consists of a darkened box, with a very small hole in one side, which projects an image from the outside world onto the opposite side. This form is often referred to as a pinhole camera. When aided by a lens, the hole in the camera doesn't have to be tiny to create a sharp and distinct image, and the exposure time can be decreased, which allows cameras to be handheld. Lenses A photographic lens is usually composed of several lens elements, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photography
Photography is the art, application, and practice of creating durable images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is employed in many fields of science, manufacturing (e.g., photolithography), and business, as well as its more direct uses for art, film and video production, recreational purposes, hobby, and mass communication. Typically, a lens is used to focus the light reflected or emitted from objects into a real image on the light-sensitive surface inside a camera during a timed exposure. With an electronic image sensor, this produces an electrical charge at each pixel, which is electronically processed and stored in a digital image file for subsequent display or processing. The result with photographic emulsion is an invisible latent image, which is later chemically "developed" into a visible image, either negative or positive, depending ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Thiosulfate
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula . Typically it is available as the white or colorless pentahydrate, . The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. Sodium thiosulfate is used in gold mining, water treatment, analytical chemistry, the development of silver-based photographic film and prints, and medicine. The medical uses of sodium thiosulfate include treatment of cyanide poisoning and pityriasis. It is on the World Health Organization's List of Essential Medicines. Uses Sodium thiosulfate is used predominantly in industry. For example, it is used to convert dyes to their soluble colorless forms, which are called leuco. It is also used to bleach "wool, cotton, silk, ...soaps, glues, clay, sand, bauxite, and... edible oils, edible fats, and gelatin." Medical uses Sodium thiosulfate is used in the treatment of cyanide poisoning. Other uses include topical treatmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para'' position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843. Production Hydroquinone is produced industrially in two main ways.Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. . * The most widely used route is similar to the cumene process in reaction mechanism and involves the dialkylation of benzene with propene to give 1,4-diisopropylbenzene. This compound reacts with air to afford the bis(hydroperoxide), which is structurally similar to cumene hydroperoxide and rearranges in acid to give acetone and hydroquino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schottky Pair
Notable people named Schottky include: * Ernst Max Schottky, botanist * Walter H. Schottky, physicist * Friedrich Schottky, mathematician Other links: * Schottky diode and Schottky barrier in electronics and physics * Schottky transistor in electronics * Schottky group In mathematics, a Schottky group is a special sort of Kleinian group, first studied by . Definition Fix some point ''p'' on the Riemann sphere. Each Jordan curve not passing through ''p'' divides the Riemann sphere into two pieces, and we c ... in mathematics * Schottky defect in condensed matter physics * Schottky anomaly in condensed matter physics * Schottky noise in electronics, described mathematically by Walter H. Schottky, and also known as shot noise {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equivalent to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V.'' Since ''q'' must be an integer multiple of the elementary charge ''e'' for any isolated particle, the gained energy in units of electronvolts conveniently equals that integer times the voltage. It is a common unit of ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frenkel Pair
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion (usually cation) leaves its place in the lattice, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion (usually the cation) is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect (does not change the over all stoichiometry of the compound). In ionic compounds, the v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frenkel Defect
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion (usually cation) leaves its place in the lattice, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion (usually the cation) is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect (does not change the over all stoichiometry of the compound). In ionic compounds, the v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latent Image
{{citations needed, date=November 2015 A latent image is an invisible image produced by the exposure to light of a photosensitive material such as photographic film. When photographic film is developed, the area that was exposed darkens and forms a visible image. In the early days of photography, the nature of the invisible change in the silver halide crystals of the film's emulsion coating was unknown, so the image was said to be "latent" until the film was treated with photographic developer. In more physical terms, a latent image is a small cluster of metallic silver atoms formed in or on a silver halide crystal due to reduction of interstitial silver ions by photoelectrons (a photolytic silver cluster). If intense exposure continues, such photolytic silver clusters grow to visible sizes. This is called ''printing out'' the image. On the other hand, the formation of a visible image by the action of photographic developer is called ''developing out'' the image. The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |