|
Secologanin
Secologanin is a secoiridoid monoterpene synthesized from geranyl pyrophosphate in the mevalonate pathway. Secologanin then proceeds with dopamine or tryptamine to form ipecac and terpene indole alkaloids, respectively. Biosynthesis Secologanin biosynthesis begins from geranyl pyrophosphate (GPP) taken from the mevalonate pathway used to make terpenoids. Recent efforts have characterized the entire secologanin biosynthetic pathway. Secologanin is formed from loganin through the action of the enzyme secologanin synthase In enzymology, a secologanin synthase (, was wrongly classified as in the past) is an enzyme that catalyzes the chemical reaction :loganin + NADPH + H+ + O2 \rightleftharpoons secologanin + NADP+ + 2 H2O The 4 substrates of this enzyme are loga .... Secologanin is then able to proceed onto produce ipecac and terpene indole alkaloids. References {{Reflist Glucosides Methyl esters Aldehydes Vinyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secologanin Synthase
In enzymology, a secologanin synthase (, was wrongly classified as in the past) is an enzyme that catalyzes the chemical reaction :loganin + NADPH + H+ + O2 \rightleftharpoons secologanin + NADP+ + 2 H2O The 4 substrates of this enzyme are loganin, NADPH, H+, and O2, whereas its 3 products are secologanin, NADP+, and H2O. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-CH group of donor with oxygen as acceptor. The systematic name of this enzyme class is loganin:oxygen oxidoreductase (ring-cleaving). This enzyme participates in indole and ipecac alkaloid Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ... biosynthesis. References * * * EC 1.14.19 NADPH-dependent enzymes Enzymes of unknown structure {{1.3-enzyme-st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Loganin
Loganin is one of the best-known of the iridoid glycosides. It is named for the Loganiaceae, having first been isolated from the seeds of a member of that plant family, namely those of ''Strychnos nux-vomica''. It also occurs in ''Alstonia boonei'' (Apocynaceae), a medicinal tree of West Africa and in the medicinal/entheogenic shrub Desfontainia spinosa (Columelliaceae) native to Central America and South America. Biosynthesis Loganin is formed from loganic acid by the enzyme loganic acid O-methyltransferase (LAMT). Loganin then becomes a substrate for the enzyme secologanin synthase (SLS) to form secologanin, a secoiridoid monoterpene found as part of ipecac and terpene indole alkaloid Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known diff ...s. References {{reflist Iridoid glycosides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secoiridoid
Iridoids are a type of monoterpenoids in the general form of cyclopentanopyran, found in a wide variety of plants and some animals. They are biosynthetically derived from 8-oxogeranial. Iridoids are typically found in plants as glycosides, most often bound to glucose. The chemical structure is exemplified by iridomyrmecin, a defensive chemical produced by the ant genus ''Iridomyrmex'', for which iridoids are named. Structurally, they are bicyclic ''cis''-fused cyclopentane-pyrans. Cleavage of a bond in the cyclopentane ring gives rise to a subclass known as ''secoiridoids'', such as oleuropein and amarogentin. Occurrence The iridoids produced by plants act primarily as a defense against herbivores or against infection by microorganisms. The variable checkerspot butterfly also contains iridoids obtained through its diet which act as a defense against avian predators. To humans and other mammals, iridoids are often characterized by a deterrent bitter taste. Aucubin and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoterpene
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranyl Pyrophosphate
Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many natural products. Occurrence GPP is an intermediate in the isoprenoid biosynthesis pathway that produces longer prenyl chains such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate as well as many terpenes. It can be prepared in the laboratory from geraniol. Related compounds * Geraniol * Farnesyl pyrophosphate * Geranylgeranyl pyrophosphate See also * Dimethylallyltranstransferase Dimethylallyltranstransferase (DMATT), also known as farnesylpyrophosphate synthase (FPPS) or as farnesyldiphosphate synthase (FDPS), is an enzyme that in humans is encoded by the FDPS gene and catalyzes the transformation of dimethylallylpyr ... References Further reading *Kulkarni RS, Pandit SS, Chidley HG, Nagel R, Schmidt A, Gershenzon J, Pujari KH, Giri AP and Gupta VS, 2013Characterization of thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mevalonate Pathway
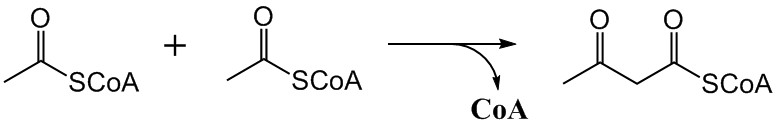
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
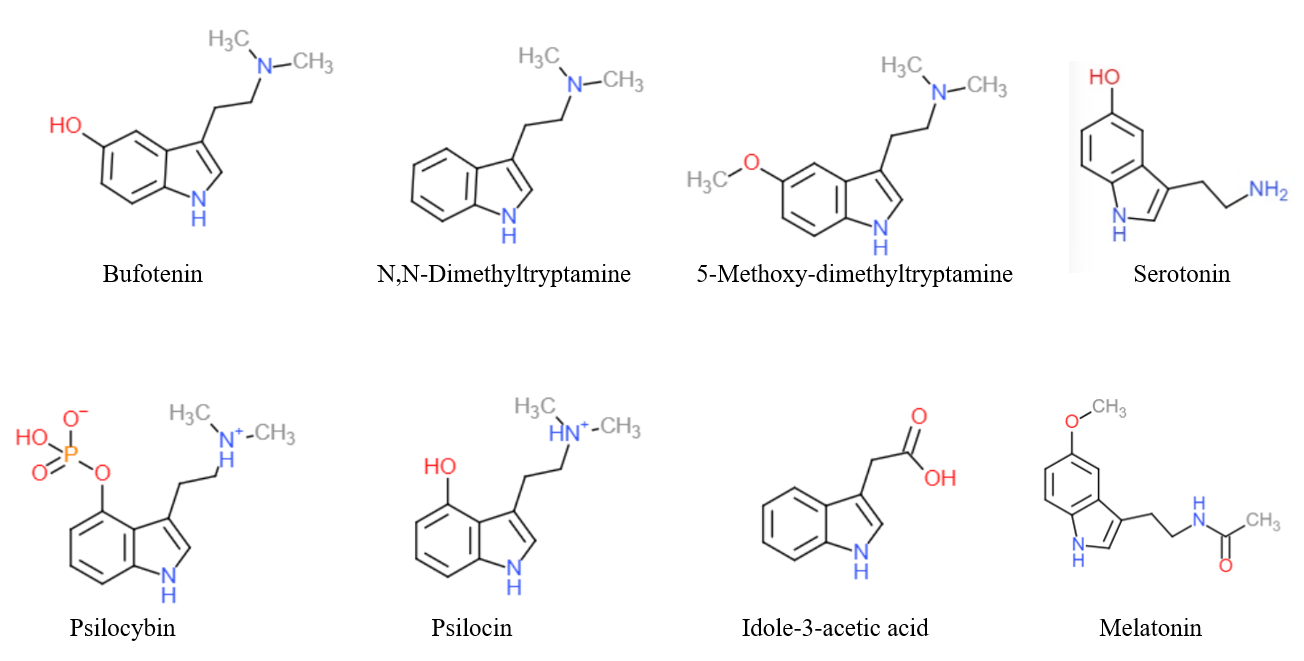
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ipecac
Syrup of ipecac (), or simply ipecac, is a drug that was once widely used as an expectorant (in low doses) and a rapid-acting emetic (in higher doses). It is obtained from the dried rhizome and roots of the ipecacuanha plant ('' Carapichea ipecacuanha''), from which it derives its name. It is no longer used in medicine. In particular, the rapidly induced forceful vomiting produced by ipecac was considered for many years to be an important front-line treatment for orally ingested poisons. However, subsequent studies (including a comprehensive 2005 meta-study) revealed the stomach purging produced by ipecac to be far less effective at lowering total body poison concentrations than the adsorption effect of oral activated charcoal (which is effective through the entire gastrointestinal tract and is often coupled with whole bowel irrigation). Ipecac also presents a small risk of overdose (being a mild poison itself) and a major risk of esophagitis and aspiration pneumonia if u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant ''Salvia div ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucosides
A glucoside is a glycoside that is derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes. The name was originally given to plant products of this nature, in which the other part of the molecule was, in the greater number of cases, an aromatic aldehydic or phenolic compound (exceptions are Jinigrin and Jalapin or Scammonin). It has now been extended to include synthetic ethers, such as those obtained by acting on alcoholic glucose solutions with hydrochloric acid, and also the polysaccharoses, e.g. cane sugar, which appear to be ethers also. Although glucose is the most common sugar present in glucosides, many are known which yield rhamnose or iso-dulcite; these may be termed pentosides. Much attention has been given to the non-sugar parts (aglyca) of the molecules; the constitutions of many have been determined, and the compounds synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Esters
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |