|
Sugar Alcohols
Sugar alcohols (also called polyhydric alcohols, polyalcohols, alditols or glycitols) are organic compounds, typically derived from sugars, containing one hydroxyl group (–OH) attached to each carbon atom. They are white, water-soluble solids that can occur naturally or be produced industrially by hydrogenation of sugars. Since they contain multiple –OH groups, they are classified as polyols. Sugar alcohols are used widely in the food industry as thickeners and sweeteners. In commercial foodstuffs, sugar alcohols are commonly used in place of table sugar (sucrose), often in combination with high-intensity artificial sweeteners, in order to offset their low sweetness. Xylitol and sorbitol are popular sugar alcohols in commercial foods. Chemical structure Sugar alcohols have the general formula HOCH2(CHOH)''n''CH2OH. In contrast, sugars have two fewer hydrogen atoms, for example HOCH2(CHOH)''n''CHO or HOCH2(CHOH)''n''−1C(O)CH2OH. The sugar alcohols differ in chain length. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylitol
Xylitol is a chemical compound with the formula , or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It can be classified as a polyalcohol and a sugar alcohol, specifically an alditol. The name derives from grc, ξύλον, ''xyl n' 'wood', with the suffix ''-itol'' used to denote sugar alcohols. Xylitol is used as a food additive and sugar substitute. Its European Union code number is E967. Replacing sugar with xylitol in food products may promote better dental health, but evidence is lacking on whether xylitol itself prevents dental cavities. History Emil Fischer, a German chemistry professor, and his assistant Rudolf Stahel isolated a new compound from beech wood chips in September 1890 and named it Xylit, the German word for xylitol. The following year, the French chemist M.G. Bertrand isolated xylitol syrup by processing wheat and oat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythritol Structure
Erythritol is an organic compound, a four-carbon sugar alcohol (or polyol) with no optical activity, used as a food additive and sugar substitute. It is naturally occurring. It can be made from corn using enzymes and fermentation. Its formula is , or HO(CH2)(CHOH)2(CH2)OH; specifically, one particular stereoisomer with that formula. Erythritol is 60–70% as sweet as sucrose (table sugar). However, erythritol is almost completely noncaloric and does not affect blood sugar or cause tooth decay. Japanese companies pioneered the commercial development of erythritol as a sweetener in the 1990s. History Erythritol was discovered in 1848 by Scottish chemist John Stenhouse and first isolated in 1852. In 1950 it was found in blackstrap molasses that was fermented by yeast, and it became commercialized as a sugar alcohol in the 1990s in Japan. Natural occurrence and production Erythritol occurs naturally in some fruit and fermented foods. It also occurs in human body fluids such as eye ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythritol
Erythritol is an organic compound, a four-carbon sugar alcohol (or polyol) with no optical activity, used as a food additive and sugar substitute. It is naturally occurring. It can be made from corn using enzymes and fermentation. Its formula is , or HO(CH2)(CHOH)2(CH2)OH; specifically, one particular stereoisomer with that formula. Erythritol is 60–70% as sweet as sucrose (table sugar). However, erythritol is almost completely noncaloric and does not affect blood sugar or cause tooth decay. Japanese companies pioneered the commercial development of erythritol as a sweetener in the 1990s. History Erythritol was discovered in 1848 by Scottish chemist John Stenhouse and first isolated in 1852. In 1950 it was found in blackstrap molasses that was fermented by yeast, and it became commercialized as a sugar alcohol in the 1990s in Japan. Natural occurrence and production Erythritol occurs naturally in some fruit and fermented foods. It also occurs in human body fluids such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorbitol
Sorbitol (), less commonly known as glucitol (), is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol group (−CH2OH). Most sorbitol is made from potato starch, but it is also found in nature, for example in apples, pears, peaches, and prunes. It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2.Kearsley, M. W.; Deis, R. C. Sorbitol and Mannitol. In Sweeteners and Sugar Alternatives in Food Technology; Ames: Oxford, 2006; pp 249-249-261. While similar, the two sugar alcohols have very different sources in nature, melting points, and uses. As an over-the-counter drug, sorbitol is used as a laxative to treat constipation. Synthesis Sorbitol may be synthesised via a glucose reduction r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribitol
Ribitol, or adonitol, is a crystalline pentose alcohol (C5H12O5) formed by the reduction of ribose. It occurs naturally in the plant ''Adonis vernalis'' as well as in the cell walls of some Gram-positive bacteria, in the form of ribitol phosphate, in teichoic acids. It also forms part of the chemical structure of riboflavin and flavin mononucleotide (FMN), which is a nucleotide coenzyme used by many enzymes, the so-called flavoproteins Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. Flavoproteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. .... References External links *GMD MS Spectrum [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arabitol
Arabitol, or arabinitol, is a sugar alcohol. It can be formed by the reduction of either arabinose or lyxose. Some organic acid tests check for the presence of D-arabitol, which may indicate overgrowth of intestinal microbes such as ''Candida albicans'' or other yeast/fungus A fungus (plural, : fungi or funguses) is any member of the group of Eukaryote, eukaryotic organisms that includes microorganisms such as yeasts and Mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified ... species. References External links * Sugar alcohols {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threitol
Threitol is a four-carbon sugar alcohol with the molecular formula C4H10O4. It is primarily used as an intermediate in the chemical synthesis of other compounds. It is the diastereomer of erythritol, which is used as a sugar substitute. In living organisms, threitol is found in the edible fungus ''Armillaria mellea''. It serves as a cryoprotectant (antifreeze agent) in the Alaskan beetle '' Upis ceramboides''. See also * Antifreeze protein * Dithiothreitol, a thiol In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ... derivative of threitol References External links * Sugar alcohols Tetroses Tetrols {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. Ethylene glycol has a sweet taste, but it is toxic in high concentrations. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation: This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetraethylene glycol. The separation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
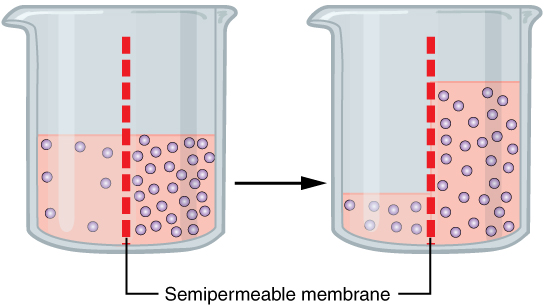
Osmotic
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semiperm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarrhea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin with loss of the normal stretchiness of the skin and irritable behaviour. This can progress to decreased urination, loss of skin color, a fast heart rate, and a decrease in responsiveness as it becomes more severe. Loose but non-watery stools in babies who are exclusively breastfed, however, are normal. The most common cause is an infection of the intestines due to either a virus, bacterium, or parasite—a condition also known as gastroenteritis. These infections are often acquired from food or water that has been contaminated by feces, or directly from another person who is infected. The three types of diarrhea are: short duration watery diarrhea, short duration bloody diarrhea, and persistent diarrhea (lasting more than two wee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |