|
Smectite Group
A smectite (; ; ) is a mineral mixture of various swelling sheet silicates (phyllosilicates), which have a three-layer 2:1 (TOT) structure and belong to the clay minerals. Smectites mainly consist of montmorillonite, but can often contain secondary minerals such as quartz and calcite. Terminology In clay mineralogy, smectite is synonym of montmorillonite (also the name of a pure clay mineral phase) to indicate a class of swelling clays. The term smectite is commonly used in Europe and in the UK while the term montmorillonite is preferred in North America, but both terms are equivalent and can be used interchangeably. For industrial and commercial applications, the term bentonite is mostly used in place of smectite or montmorillonite. Mineralogical structure The 2:1 layer (TOT) structure consists of two silica (SiO2) tetrahedral (T) layers which are electrostatically cross-linked via an Al2O3 ( gibbsite), or Fe2O3, octahedral (O) central layer. The TOT elementary layers are n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clay Magnified
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolinite, ). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impurities, such as a reddish or brownish colour from small amounts of iron oxide. Clays develop plasticity when wet but can be hardened through firing. Clay is the longest-known ceramic material. Prehistoric humans discovered the useful properties of clay and used it for making pottery. Some of the earliest pottery shards have been dated to around 14,000 BCE, and clay tablets were the first known writing medium. Clay is used in many modern industrial processes, such as paper making, cement production, and chemical filtering. Between one-half and two-thirds of the world's population live or work in buildings made with clay, often baked into brick, as an essential part of its load-bearing structure. In agriculture, clay content is a majo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volcanic Glass
Volcanic glass is the amorphous (uncrystallized) product of rapidly cooling magma. Like all types of glass, it is a state of matter intermediate between the closely packed, highly ordered array of a crystal and the highly disordered array of liquid. Volcanic glass may refer to the interstitial material, or matrix, in an aphanitic (fine-grained) volcanic rock, or to any of several types of vitreous igneous rocks. Origin Volcanic glass is formed when magma is rapidly cooled. Magma rapidly cooled to below its normal crystallization temperature becomes a supercooled liquid, and, with further rapid cooling, this becomes an amorphous solid. The change from supercooled liquid to glass occurs at a temperature called the glass transition temperature, which depends on both cooling rate and the amount of water dissolved in the magma. Magma rich in silica and poor in dissolved water is most easily cooled rapidly enough to form volcanic glass. As a result, rhyolite magmas, which are high in si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
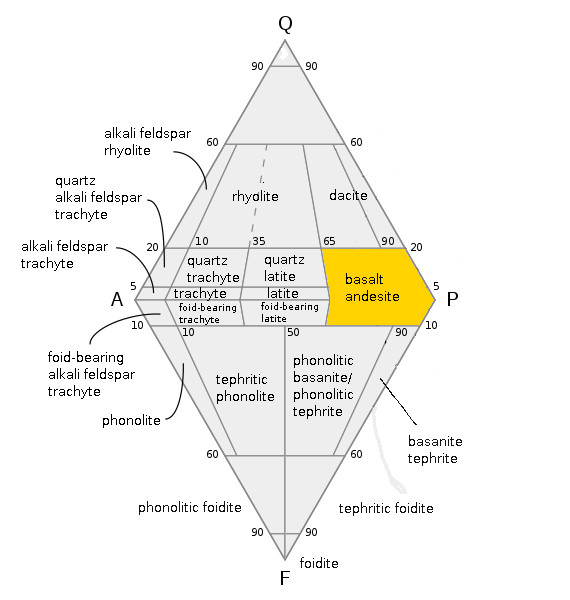
Gabbro
Gabbro ( ) is a phaneritic (coarse-grained and magnesium- and iron-rich), mafic intrusive igneous rock formed from the slow cooling magma into a holocrystalline mass deep beneath the Earth's surface. Slow-cooling, coarse-grained gabbro is chemically equivalent to rapid-cooling, fine-grained basalt. Much of the Earth's oceanic crust is made of gabbro, formed at mid-ocean ridges. Gabbro is also found as plutons associated with continental volcanism. Due to its variant nature, the term ''gabbro'' may be applied loosely to a wide range of intrusive rocks, many of which are merely "gabbroic". By rough analogy, gabbro is to basalt as granite is to rhyolite. Etymology The term "gabbro" was used in the 1760s to name a set of rock types that were found in the ophiolites of the Apennine Mountains in Italy. It was named after Gabbro, a hamlet near Rosignano Marittimo in Tuscany. Then, in 1809, the German geologist Christian Leopold von Buch used the term more restrictively in his d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basalt
Basalt (; ) is an aphanite, aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron (mafic lava) exposed at or very near the planetary surface, surface of a terrestrial planet, rocky planet or natural satellite, moon. More than 90% of all volcanic rock on Earth is basalt. Rapid-cooling, fine-grained basalt is chemically equivalent to slow-cooling, coarse-grained gabbro. The eruption of basalt lava is observed by geologists at about 20 volcanoes per year. Basalt is also an important rock type on other planetary bodies in the Solar System. For example, the bulk of the plains of volcanism on Venus, Venus, which cover ~80% of the surface, are basaltic; the lunar mare, lunar maria are plains of flood-basaltic lava flows; and basalt is a common rock on the surface of Mars. Molten basalt lava has a low viscosity due to its relatively low silica content (between 45% and 52%), resulting in rapidly moving lava flo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no movement), and so is distinct from erosion, which involves the transport of rocks and minerals by agents such as water, ice, snow, wind, waves and gravity. Weathering processes are either physical or chemical. The former involves the breakdown of rocks and soils through such mechanical effects as heat, water, ice and wind. The latter covers reactions to water, atmospheric gases and biologically produced chemicals with rocks and soils. Water is the principal agent behind both kinds, though atmospheric oxygen and carbon dioxide and the activities of biological organisms are also important. Biological chemical weathering is also called biological weathering. The materials left after the rock breaks down combine with organic material to create so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally, but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. Character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation-exchange Capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be retained on soil particle surfaces. Negative charges on the surfaces of soil particles bind positively-charged atoms or molecules (cations), but allow these to exchange with other positively charged particles in the surrounding soil water. This is one of the ways that solid materials in soil alter the chemistry of the soil. CEC affects many aspects of soil chemistry, and is used as a measure of soil fertility, as it indicates the capacity of the soil to retain several nutrients (e.g. K+, NH4+, Ca2+) in plant-available form. It also indicates the capacity to retain pollutant cations (e.g. Pb2+). Definition and principles Cation-exchange capacity is defined as the amount of positive charge that can be exchanged per mass of soil, usually measured in cmolc/kg. Some texts use the older, equivalent units me/100g or meq/100g. CEC is measured in moles of electric charge, so a cation-exchange capacity of 10 cmolc/kg co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Illite
Illite, also called hydromica or hydromuscovite, is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina octahedron (O) – silica tetrahedron (T) layers. The space between this T-O-T sequence of layers is occupied by poorly hydrated potassium cations which are responsible for the absence of swelling. Structurally, illite is quite similar to muscovite with slightly more silicon, magnesium, iron, and water and slightly less tetrahedral aluminium and interlayer potassium. The chemical formula is given as , but there is considerable ion (isomorphic) substitution. It occurs as aggregates of small monoclinic grey to white crystals. Due to the small size, positive identification usually requires x-ray diffraction or SEM-EDS ( automated mineralogy) analysis. Illite occurs as an altered product of muscovite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create cation, an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-flame color, colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vermiculite
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliation occurs when the mineral is heated sufficiently; commercial furnaces can routinely produce this effect. Vermiculite forms by the weathering or hydrothermal alteration of biotite or phlogopite. http://www.mindat.org/min-4170.html Mindat.org Large commercial vermiculite mines exist in the United States, Russia, South Africa, China, and Brazil. Occurrence Vermiculite was first described in 1824 for an occurrence in Millbury, Massachusetts. Its name is from the Latin , "to breed worms", for the manner in which it exfoliates when heated. It typically occurs as an alteration product at the contact between felsic and mafic or ultramafic rocks such as pyroxenites and dunites. It also occurs in carbonatites and metamorphosed magnesium-rich limestone. Associated mineral phases include: corundum, apatite, serpentine, and talc. It occurs interlayered with chlorite, biotite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |