|
Silver Bromide
Silver bromide (AgBr), a soft, pale-yellow, water-insoluble salt well known (along with other silver halides) for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for faking the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite (bromyrite). Preparation Although the compound can be found in mineral form, AgBr is typically prepared by the reaction of silver nitrate with an alkali bromide, typically potassium bromide: :AgNO3(aq) + KBr(aq) → AgBr(s)+ KNO3(aq) Although less convenient, the salt can also be prepared directly from its elements. Modern preparation of a simple, light-sensitive surface involves forming an emulsion of silver halide crystals in a gelatine, which is then coated onto a film or other support. The crystals are formed by precipitation in a controlled environment to prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromargyrite
Bromyrite or bromargyrite is a natural mineral form of silver bromide found mainly in Mexico and Chile. Hardness is 1.5 to 2. Related are chlorargyrite and iodyrite. It was first described in 1859 for an occurrence in Plateros, Zacatecas, Mexico where it occurred in a silver deposit as an oxidation product of primary ore minerals. It occurs in arid environments along with native silver, iodargyrite and smithsonite along with iron and manganese oxide minerals. References Bromides Silver minerals Halide minerals Cubic minerals Minerals in space group 225 {{Halide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride Crystal
Sodium is a chemical element; it has symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including humans. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromides
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm. Natural occurrence Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper. Formation and re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Halides
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are Ionic compound, ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride. File:NaCl polyhedra.svg, Sodium chloride crystal structure File:Uranium-hexafluoride-unit-cell-3D-balls.png, Discrete UF6 molecules File:Alpha-palladium(II)-chloride-xtal-3D-balls.png, Infinite chains of one form of palladium chloride Preparation The halogens can all react with metals to form metal halides according to the following equation: :2M + nX2 → 2MXn where M is the metal, X is the halogen, and MXn is the metal halide. In practice, this type of reaction may be very exothermic, hence impractical as a preparative technique. Additionally, many transition metals can adopt multiple oxidation states, which complicates matters. As the halogens are strong oxidizers, direct combination of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
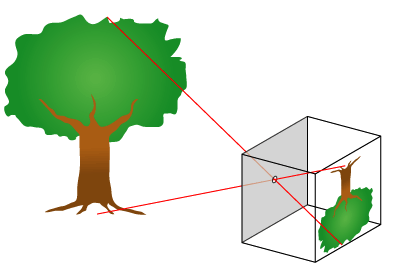
Science Of Photography
The science of photography is the use of chemistry and physics in all aspects of photography. This applies to the camera, its lenses, physical operation of the camera, electronic camera internals, and the process of developing Photographic film, film in order to take and develop pictures properly. Optics Camera obscura The fundamental technology of most photography, whether digital or analog, is the camera obscura effect and its ability to transform of a three dimensional scene into a two dimensional image. At its most basic, a camera obscura consists of a darkened box, with a very small hole in one side, which projects an image from the outside world onto the opposite side. This form is often referred to as a pinhole camera. When aided by a lens, the hole in the camera doesn't have to be tiny to create a sharp and distinct image, and the exposure time can be decreased, which allows cameras to be handheld. Lenses A photographic lens is usually composed of several lens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photography
Photography is the visual arts, art, application, and practice of creating images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is employed in many fields of science, manufacturing (e.g., photolithography), and business, as well as its more direct uses for art, film and video production, recreational purposes, hobby, and mass communication. A person who operates a camera to capture or take Photograph, photographs is called a photographer, while the captured image, also known as a photograph, is the result produced by the camera. Typically, a lens is used to focus (optics), focus the light reflected or emitted from objects into a real image on the light-sensitive surface inside a camera during a timed Exposure (photography), exposure. With an electronic image sensor, this produces an Charge-coupled device, electrical charge at each pixel, which is Image processing, electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Thiosulfate
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula . Typically it is available as the white or colorless pentahydrate (x = 5), which is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications. Uses Sodium thiosulfate is used predominantly in dyeing. It converts some dyes to their soluble colorless "leuco" forms. It is also used to bleach "wool, cotton, silk, ...soaps, glues, clay, sand, bauxite, and... edible oils, edible fats, and gelatin." Medical uses Sodium thiosulfate is used in the treatment of cyanide poisoning. It is on the World Health Organization's List of Essential Medicines. Other uses include topical treatment of ringworm and tinea versicolor, and treating some side effects of hemodialysis and chemotherapy. In September 2022, the U.S. Food and Drug Administration (FDA) approved sodium thiosulfate under the trade name Pedmark to lessen the risk of oto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para'' position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843. In 2022, it was the 268th most commonly prescribed medication in the United States, with more than 900,000 prescriptions. Production Hydroquinone is produced industrially in two main ways.Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. . * The most widely used route is similar to the cumene process in reaction mechanism and involves the dialkylation of benzene with propene to give 1,4-diisopropylbenzene. This compound reacts with air to afford the bis(hydrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schottky Pair
Notable people named Schottky include: * Ernst Max Schottky, botanist * Walter H. Schottky, physicist * Friedrich Schottky, mathematician Other links: * Schottky diode and Schottky barrier in electronics and physics * Schottky transistor in electronics * Schottky group in mathematics * Schottky defect in condensed matter physics * Schottky anomaly in condensed matter physics * Schottky noise in electronics, described mathematically by Walter H. Schottky, and also known as shot noise {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum. When used as a Units of energy, unit of energy, the numerical value of 1 eV in joules (symbol J) is equal to the numerical value of the Electric charge, charge of an electron in coulombs (symbol C). Under the 2019 revision of the SI, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in Particle accelerator#Electrostatic particle accelerators, electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V''. Definition and use An electronvolt is the amount of energy gained or lost by a single electron when it moves through an Voltage, electric potential differenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frenkel Pair
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion (usually cation) leaves its place in the structure, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion (usually the cation) is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect (does not change the over all stoichiometry of the compound). In ionic compounds, the vacancy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frenkel Defect
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion (usually cation) leaves its place in the structure, creating a vacancy and becomes an interstitial by lodging in a nearby location. In elemental systems, they are primarily generated during particle irradiation, as their formation enthalpy is typically much higher than for other point defects, such as vacancies, and thus their equilibrium concentration according to the Boltzmann distribution is below the detection limit. In ionic crystals, which usually possess low coordination number or a considerable disparity in the sizes of the ions, this defect can be generated also spontaneously, where the smaller ion (usually the cation) is dislocated. Similar to a Schottky defect the Frenkel defect is a stoichiometric defect (does not change the over all stoichiometry of the compound). In ionic compounds, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |