|
Scavenger Receptor (immunology)
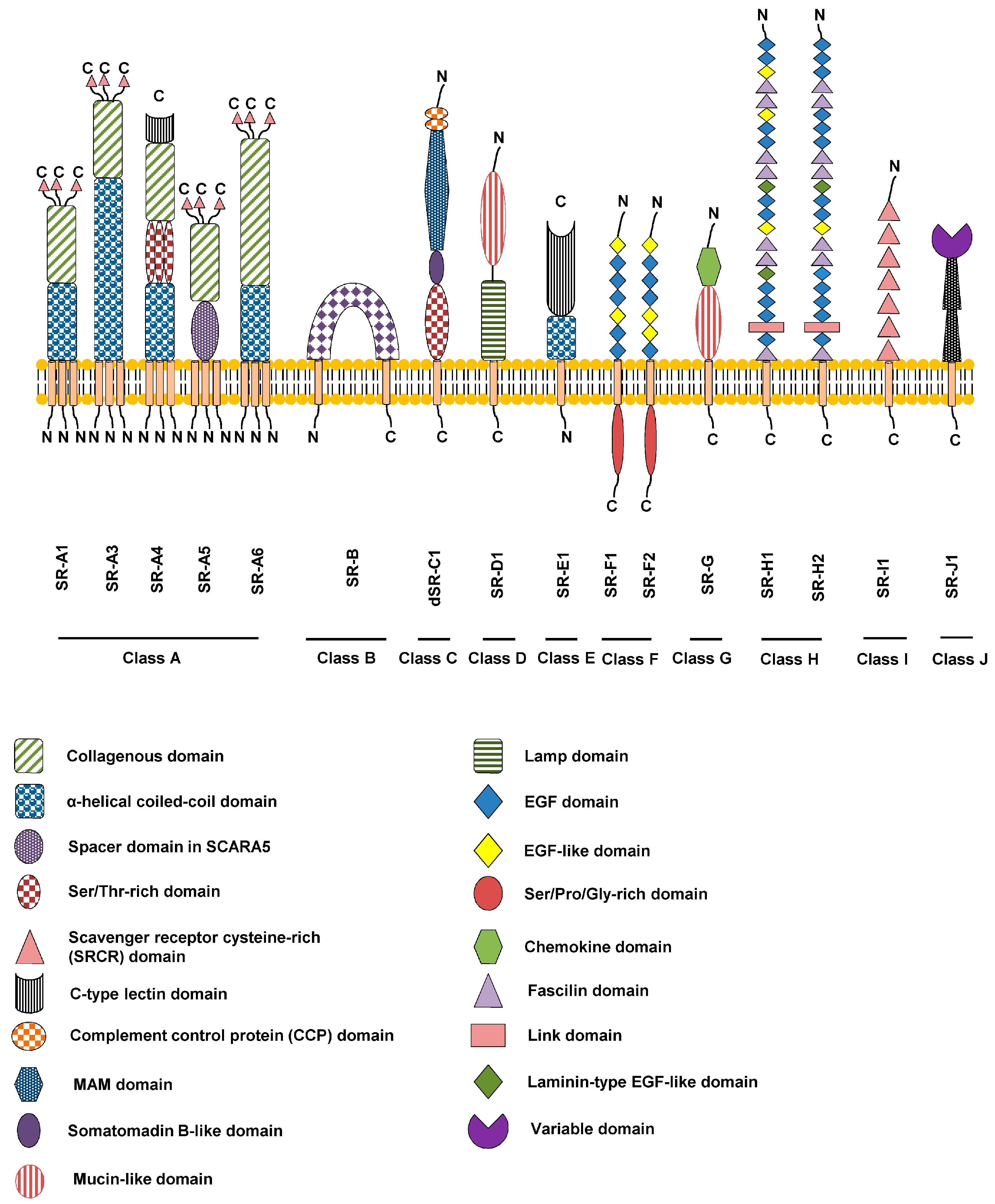
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns (PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A1, SR-A1.1, and MARCO (SR-A6). Function The scavenger receptor superfamily is defined by its ability to recognize and bind a broad range of common ligands. These ligands include: polyanionic ligands including lipoproteins, apoptotic cells, choleste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Superfamily
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred (see homology (biology), homology). Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence similarity is evident. Sequence homology can then be deduced even if not apparent (due to low sequence similarity). Superfamilies typically contain several protein families which show sequence similarity within each family. The term ''protein clan'' is commonly used for protease and glycosyl hydrolases superfamilies based on the MEROPS and CAZy classification systems. Identification Superfamilies of proteins are identified using a number of methods. Closely related members can be identified by different methods to those needed to group the most evolutionarily divergent members. Sequence similarity Historically, the similarity of different amino acid sequences has been the most common method of inferring Sequence homology, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
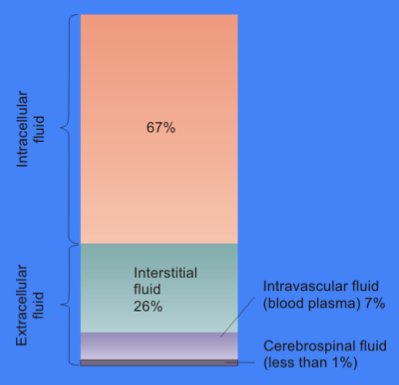
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl radical (OH.), and singlet oxygen(1O2). ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations. Inventory of ROS ROS are not uniformly defined. All sources include superoxide, singlet oxygen, and hydroxyl radical. Hydrogen peroxide is not nearly as reactive as these s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothelium
The endothelium (: endothelia) is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells in direct contact with blood are called vascular endothelial cells whereas those in direct contact with lymph are known as lymphatic endothelial cells. Vascular endothelial cells line the entire circulatory system, from the heart to the smallest capillaries. These cells have unique functions that include fluid filtration, such as in the glomerulus of the kidney, blood vessel tone, hemostasis, neutrophil recruitment, and hormone trafficking. Endothelium of the interior surfaces of the heart chambers is called endocardium. An impaired function can lead to serious health issues throughout the body. Structure The endothelium is a thin layer of single flat (squamous) cells that line the inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MSR1
Macrophage scavenger receptor 1, also known as MSR1, is a protein which in humans is encoded by the ''MSR1'' gene. MSR1 has also been designated CD204 (cluster of differentiation 204). Function This gene encodes the class A macrophage scavenger receptors, which include three different types (1, 2, 3) generated by alternative splicing of this gene. These receptors or isoforms are trimeric integral membrane glycoproteins and have been implicated in many macrophage-associated physiological and pathological processes including atherosclerosis, Alzheimer's disease, and host defense. They were thought to be expressed macrophage-specific, but recently shown to be present on different dendritic cells classes, too. The isoforms type 1 and type 2 are functional receptors and are able to mediate the endocytosis of modified low density lipoproteins (LDLs). The isoform type 3 does not internalize modified LDL (acetyl-LDL) despite having the domain shown to mediate this function in the type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
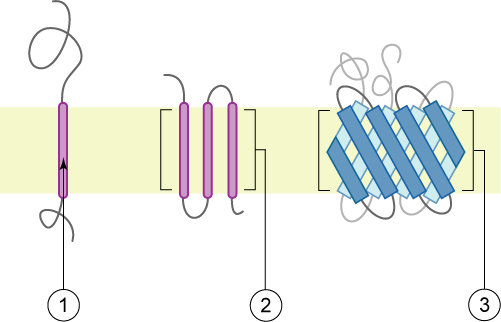
Bitopic Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. More than 2300 single-pass membrane proteins were identified in the human genome. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminal End
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic acid, carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in Writing system#Directionality, LTR writing systems). This correlates the translation (biology), translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one anot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. LCysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Ancient Greek, Greek κύστις ''kýstis'', "bladder". The thiol is susceptible to oxidation to give the disulfide bond, disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. The deprotonated form can generally be described by the symbol Cym as well. When used as a food additive, cysteine has the E number E920. Cysteine is Genetic code, encoded by the codo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of biomineralization, mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the Gut (anatomy), gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |