|
Reverse Diffusion
Reverse diffusion refers to a situation where the transport of particles (atoms or molecules) in a medium occurs towards regions of higher concentration gradients, opposite to that observed during diffusion. This phenomenon occurs during phase separation and is described by the Cahn–Hilliard equation. Reverse diffusion also refers to when water is forced from a region of lower concentration to high. It can occur in osmosis Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively permeable membrane, selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of .... See also References Diffusion {{Physics-stub cs:Reverzní osmóza ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing. The concept of diffusion is widely used in many fields, including physics (Molecular diffusion, particle diffusion), chemistry, biology, sociology, economics, statistics, data science, and finance (diffusion of people, ideas, data and price v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is a different material, in its own separate phase. (See .) More precisely, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter (as where oil and water separate into distinct phases, both in the liquid state). Types of phases Distinct phases may be described as different states of matter such as gas, liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cahn–Hilliard Equation
The Cahn–Hilliard equation (after John W. Cahn and John E. Hilliard) is an equation of mathematical physics which describes the process of Phase (matter), phase separation, spinodal decomposition, by which the two components of a binary fluid spontaneously separate and form domains pure in each component. If c is the concentration of the fluid, with c=\pm1 indicating domains, then the equation is written as :\frac = D\nabla^2\left(c^3-c-\gamma\nabla^2 c\right), where D is a diffusion coefficient with units of \text^2/\text and \sqrt gives the length of the transition regions between the domains. Here \partial/ is the partial time derivative and \nabla^2 is the Laplacian in n dimensions. Additionally, the quantity \mu = c^3-c-\gamma\nabla^2 c is identified as a chemical potential. It is related to the Allen–Cahn equation, as well as the stochastic Allen–Cahn and the stochastic Cahn–Hilliard equations. Features and applications Of interest to mathematicians is the exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
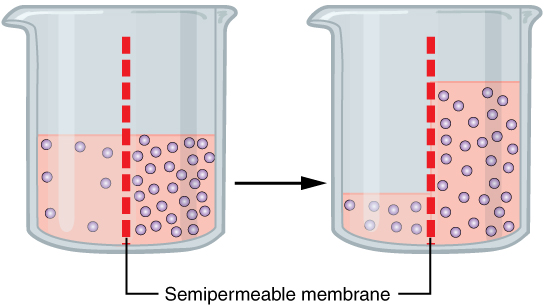
Osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively permeable membrane, selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do Work (physics), work. Osmotic pressure is defined as the external pressure required to prevent net movement of solvent across the membrane. Osmotic pressure is a colligative properties, colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biology, bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing. The concept of diffusion is widely used in many fields, including physics (Molecular diffusion, particle diffusion), chemistry, biology, sociology, economics, statistics, data science, and finance (diffusion of people, ideas, data and price v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |