|
Redlich–Kwong Equation Of State
In physics and thermodynamics, the Redlich–Kwong equation of state is an empirical, algebraic equation that relates temperature, pressure, and volume of gases. It is generally more accurate than the van der Waals equation and the ideal gas equation at temperatures above the critical temperature. It was formulated by Otto Redlich and Joseph Neng Shun Kwong in 1949. It showed that a two-parameter, cubic equation of state could well reflect reality in many situations, standing alongside the much more complicated Beattie–Bridgeman model and Benedict–Webb–Rubin equation that were used at the time. Although it was initially developed for gases, the Redlich–Kwong equation has been considered the most modified equation of state since those modifications have been aimed to generalize the predictive results obtained from it. Although this equation is not currently employed in practical applications, modifications derived from this mathematical model like the Soave Redlich-Kwo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." It is one of the most fundamental scientific disciplines. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. (...) You will come to see physics as a towering achievement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
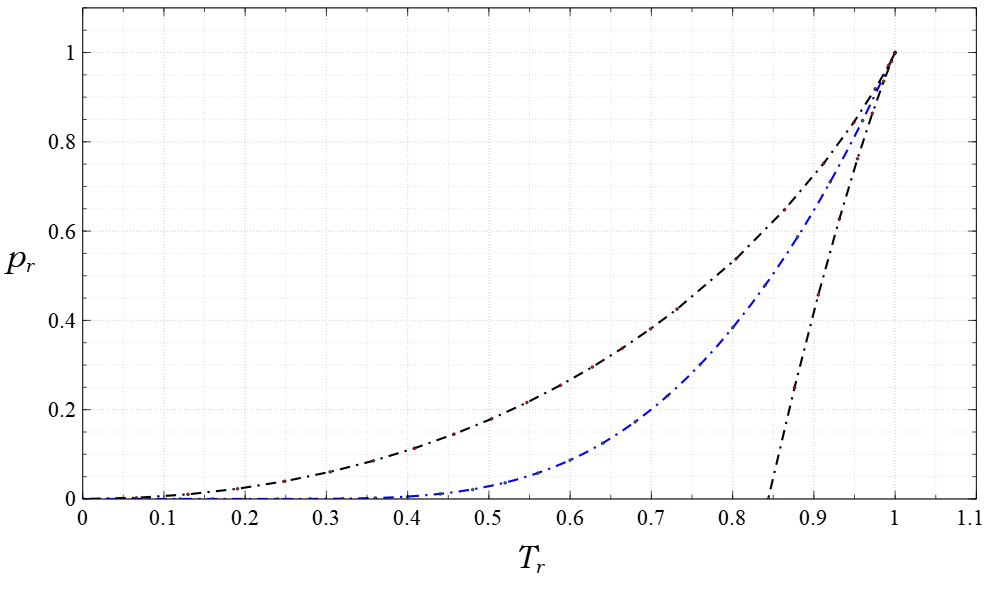
Compressibility Factor
In thermodynamics, the compressibility factor (Z), also known as the compression factor or the gas deviation factor, describes the deviation of a real gas from ideal gas behaviour. It is simply defined as the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behaviour.Properties of Natural Gases . Includes a chart of compressibility factors versus reduced pressure and reduced temperature (on last page of the PDF document) In general, deviation from ideal behaviour becomes more significant the closer a gas is to a phase change, the lower the temperat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equation Of State
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Though there are many equations of state, none accurately predicts properties of substances under all conditions. The quest for a universal equation of state has spanned three centuries. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fugacity
In thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of chemical equilibrium. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas. Fugacities are determined experimentally or estimated from various models such as a Van der Waals gas that are closer to reality than an ideal gas. The real gas pressure and fugacity are related through the dimensionless fugacity coefficient \varphi = \frac. For an ideal gas, fugacity and pressure are equal, and so . Taken at the same temperature and pressure, the difference between the molar Gibbs free energies of a real gas and the corresponding ideal gas is equal to . The fugacity is closely related to the thermodynamic activity. For a gas, the activity is simply the fugacity divided by a reference pressure to give a dimensionless quantity. This reference pressure is called the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acentric Factor
The acentric factor is a conceptual number introduced by Kenneth Pitzer in 1955, proven to be useful in the description of fluids. It has become a standard for the phase characterization of single and pure components, along with other state description parameters such as molecular weight, critical temperature, critical pressure, and critical volume (or critical compressibility). The acentric factor is also said to be a measure of the non-sphericity (centricity) of molecules. Pitzer defined from the relationship : \omega = -\log_(p^\text_\text) - 1 \text T_\text = 0.7, where p^\text_\text = p^\text / p_c is the reduced saturation vapor pressure, and T_\text = T / T_c is the reduced temperature. Pitzer developed this factor by studying the vapor-pressure curves of various pure substances. Thermodynamically, the vapor-pressure curve for pure components can be mathematically described using the Clausius–Clapeyron equation. The integrated form of equation is mainly used fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Chemical Society
The American Chemical Society (ACS) is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 155,000 members at all degree levels and in all fields of chemistry, chemical engineering, and related fields. It is one of the world's largest scientific societies by membership. The ACS is a 501(c) organization, 501(c)(3) non-profit organization and holds a congressional charter under Title 36 of the United States Code. Its headquarters are located in Washington, D.C., and it has a large concentration of staff in Columbus, Ohio. The ACS is a leading source of scientific information through its peer-reviewed scientific journals, national conferences, and the Chemical Abstracts Service. Its publications division produces over 80 Scientific journal, scholarly journals including the prestigious ''Journal of the American Chemical Society'', as well as the weekly tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Portland, Oregon
Portland ( ) is the List of cities in Oregon, most populous city in the U.S. state of Oregon, located in the Pacific Northwest region. Situated close to northwest Oregon at the confluence of the Willamette River, Willamette and Columbia River, Columbia rivers, it is the county seat of Multnomah County, Oregon, Multnomah County, Oregon's most populous county. Portland's population was 652,503, making it the List of United States cities by population, 28th most populous city in the United States, the sixth most populous on the West Coast of the United States, West Coast, and the third most populous in the Pacific Northwest after Seattle and Vancouver. Approximately 2.5 million people live in the Portland metropolitan area, Oregon, Portland metropolitan area, making it the List of metropolitan statistical areas, 26th most populous in the United States. Almost half of Oregon's population resides within the Portland metro area. Named after Portland, Maine, which is itself named aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emeryville, California
Emeryville is a city located in northwest Alameda County, California, in the United States. It lies in a corridor between the cities of Berkeley, California, Berkeley and Oakland, California, Oakland, with a border on the shore of San Francisco Bay. The resident population was 12,905 as of 2020. Its proximity to San Francisco, the San Francisco–Oakland Bay Bridge, Bay Bridge, the University of California, Berkeley, and Silicon Valley has been a catalyst for recent economic growth. It is the home to Pixar, Pixar Animation Studios, Peet's Coffee, the Center for Investigative Reporting, Alternative Tentacles and Clif Bar. In addition, several well-known tech and software companies are located in Emeryville: LeapFrog Enterprises, LeapFrog, Sendmail, Inc., Sendmail, MobiTV, Novartis (formerly Chiron Corporation, Chiron before April 2006), and HCL BigFix, BigFix (now HCL). Emeryville attracts many weekday commuters due to its position as a regional employment center. Emeryville ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shell Development Emeryville
The Emeryville Research Center of Shell Development Company in Emeryville, California was a major research facility of Shell Oil Company in the United States from 1928 until 1972, when Shell Development relocated to Houston, Texas."Research in physics at the Emeryville Research Center of Shell Development Company, 1928-1966, 1966," by Shell Development Company. Call Number: IH190 54 pages. Owning Repository: American Institute of Physics. Center for History of Physics. Niels Bohr Library. One Physics Ellipse, College Park, MD 20740, USA Shell Development's Emeryville facilities were located on about , included nearly 90 buildings at its peak, and when decommissioned in 1972, employed a staff of about 1500. Inventions, technical contributions, resources Tricresyl phosphate (TCP) and other gasoline additives were developed at Emeryville. Tar sands extraction and other techniques to increase oil reserves were studied at bench scale and in pilot plants. Shell Development also pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johannes Diderik Van Der Waals
Johannes Diderik van der Waals (; 23 November 1837 – 8 March 1923) was a Dutch theoretical physicist who received the Nobel Prize in Physics in 1910 "for his work on the equation of state for gases and liquids". Van der Waals started his career as a schoolteacher. He became the first physics professor of the University of Amsterdam when its status was upgraded to Municipal University in 1877. His name is primarily associated with the van der Waals equation, an equation of state that describes the behavior of gases and their condensation to the liquid phase. His name is also associated with van der Waals forces (forces between stable molecules), with van der Waals molecules (small molecular clusters bound by van der Waals forces), and with the van der Waals radius (size of molecules). James Clerk Maxwell once said that, "there can be no doubt that the name of Van der Waals will soon be among the foremost in molecular science." In his 1873 thesis, Van der Waals noted the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Equation
The van der Waals equation is a mathematical formula that describes the behavior of real gases. It is an equation of state that relates the pressure, volume, Avogadro's law, number of molecules, and temperature in a fluid. The equation modifies the ideal gas law in two ways: first, it considers particles to have a finite diameter (whereas an ideal gas consists of point particles); second, its particles interact with each other (unlike an ideal gas, whose particles move as though alone in the volume). The equation is named after Dutch physicist Johannes Diderik van der Waals, who first derived it in 1873 as part of his doctoral thesis. Van der Waals based the equation on the idea that fluids are composed of discrete particles, which few scientists believed existed. However, the equation accurately predicted the behavior of a fluid around its Critical point (thermodynamics), critical point, which had been discovered a few years earlier. Its qualitative and quantitative agreement w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole Fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the total amount of all constituents in a mixture, ''n''tot (also expressed in moles): :x_i = \frac It is denoted ''xi'' (lowercase Roman letter '' x''), sometimes (lowercase Greek letter chi). (For mixtures of gases, the letter ''y'' is recommended.) It is a dimensionless quantity with dimension of \mathsf/\mathsf and dimensionless unit of moles per mole (mol/mol or molmol−1) or simply 1; metric prefixes may also be used (e.g., nmol/mol for 10−9). When expressed in percent, it is known as the mole percent or molar percentage (unit symbol %, sometimes "mol%", equivalent to cmol/mol for 10−2). The mole fraction is called amount fraction by the International Union of Pure and Applied Chemistry (IUPAC) and amount-of-substance fractio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |