|
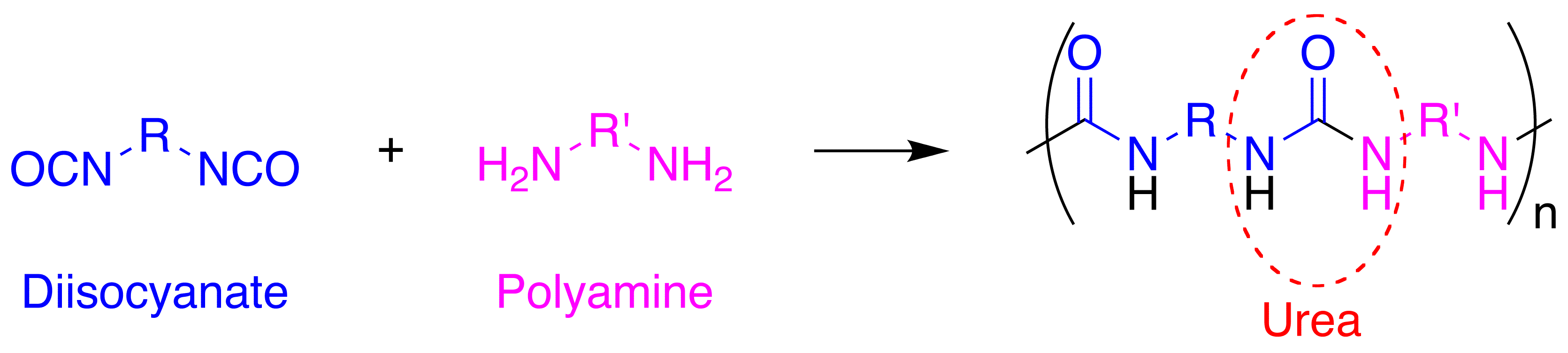
Polyurea
Polyurea is a type of elastomer that is derived from the reaction product of an isocyanate component and a synthetic resin blend component through step-growth polymerization. The isocyanate can be aromatic or aliphatic in nature. It can be monomer, polymer, or any variant reaction of isocyanates, quasi-prepolymer or a prepolymer. The prepolymer, or quasi-prepolymer, can be made of an amine-terminated polymer resin, or a hydroxyl-terminated polymer resin. The resin blend may be made up of amine-terminated polymer resins, and/or amine-terminated chain extenders. The amine-terminated polymer resins do not have any intentional hydroxyl moieties. Any hydroxyls are the result of incomplete conversion to the amine-terminated polymer resins. The resin blend may also contain additives or non-primary components. These additives may contain hydroxyls, such as pre-dispersed pigments in a polyol carrier. Normally, the resin blend does not contain a catalyst(s). Polymer structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurea
Polyurea is a type of elastomer that is derived from the reaction product of an isocyanate component and a synthetic resin blend component through step-growth polymerization. The isocyanate can be aromatic or aliphatic in nature. It can be monomer, polymer, or any variant reaction of isocyanates, quasi-prepolymer or a prepolymer. The prepolymer, or quasi-prepolymer, can be made of an amine-terminated polymer resin, or a hydroxyl-terminated polymer resin. The resin blend may be made up of amine-terminated polymer resins, and/or amine-terminated chain extenders. The amine-terminated polymer resins do not have any intentional hydroxyl moieties. Any hydroxyls are the result of incomplete conversion to the amine-terminated polymer resins. The resin blend may also contain additives or non-primary components. These additives may contain hydroxyls, such as pre-dispersed pigments in a polyol carrier. Normally, the resin blend does not contain a catalyst(s). Polymer structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibers such as spandex and PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016. A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as alternating copolymers. Both the isocyanates and polyols used to make a polyurethane contain two or more functional groups per molecule. Global production in 2019 wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prepolymer
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully cured, high-molecular-mass state. As such, mixtures of reactive polymers with un-reacted monomers may also be referred to as pre-polymers. The term "pre-polymer" and "polymer precursor" may be interchanged. Polyurethane and polyurea prepolymers In polyurethane chemistry, prepolymers and oligomers are frequently produced and then further formulated into CASE applications - Coatings, Adhesives, Sealants, and Elastomers. An isocyanate (usually a diisocyanate) is reacted with a polyol. All types of polyol may in theory be used to produce polyurethane prepolymers. These then find use in CASE applications. When polyurethane dispersions are synthesized, a prepolymer is first produced usually modified with DMPA. In polyurea prepolymer production ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C-N=C angle is 134.9° and the N=C=O angle is 173.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyol
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups (). The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively. Classification Polyols may be classified according to their chemistry. Some of these chemistries are polyether, polyester, polycarbonate and also acrylic polyols. Polyether polyols may be further subdivided and classified as polyethylene oxide or polyethylene glycol (PEG), polypropylene glycol (PPG) and Polytetrahydrofuran or PTMEG. These have 2, 3 and 4 carbons respectively per oxygen atom in the repeat unit. Polycaprolactone polyols are also commercially available. There is also an increasing trend to use biobased (and hence renewable) polyols. Uses Polyether polyols have numerous uses. As an example, polyurethane foam is a big user of polyether polyols. Po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spandex
Spandex, Lycra, or elastane is a synthetic fiber known for its exceptional elasticity. It is a polyether-polyurea copolymer that was invented in 1958 by chemist Joseph Shivers at DuPont's Benger Laboratory in Waynesboro, Virginia, US. The generic name "spandex", which is an anagram of the word "expands", is the preferred name in North America. In continental Europe, it is referred to by variants of "elastane", including (France), (Germany, Sweden), (Spain), (Italy), and (Netherlands); and in the UK, Ireland, Portugal, Spain, Latin America, Australia, and New Zealand, it is primarily known as "Lycra". Brand names for spandex include Lycra (made by The Lycra Company, previously a division of DuPont Textiles and Interiors), Elaspan (The Lycra Company), Acepora (Taekwang Group), Creora (Hyosung), INVIYA ( Indorama Corporation), ROICA and Dorlastan (Asahi Kasei), Linel (Fillattice), and ESPA ( Toyobo). History In the post-World War II era, DuPont Textiles Fibers Departm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamic Acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields an equal amount of ammonium carbamate . The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.J. B. Bossa, P. Theulé, F. Duvernay, F. Borget and T. Chiavassa (2008): "Carbamic acid and carbamate formation in NH3:CO2 ices – UV irradiation versus thermal processes". ''Astronomy and Astrophysics'', volume 492, issue 3, pages 719-724. Carbamic acid could be seen as both an amine and carboxylic acid, and therefore an amino acid;R. K. Khanna and M. H. Moore (1999): "Carbamic acid: molecular structure and IR spectra". ''Spectrochimica Acta Part A: M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
