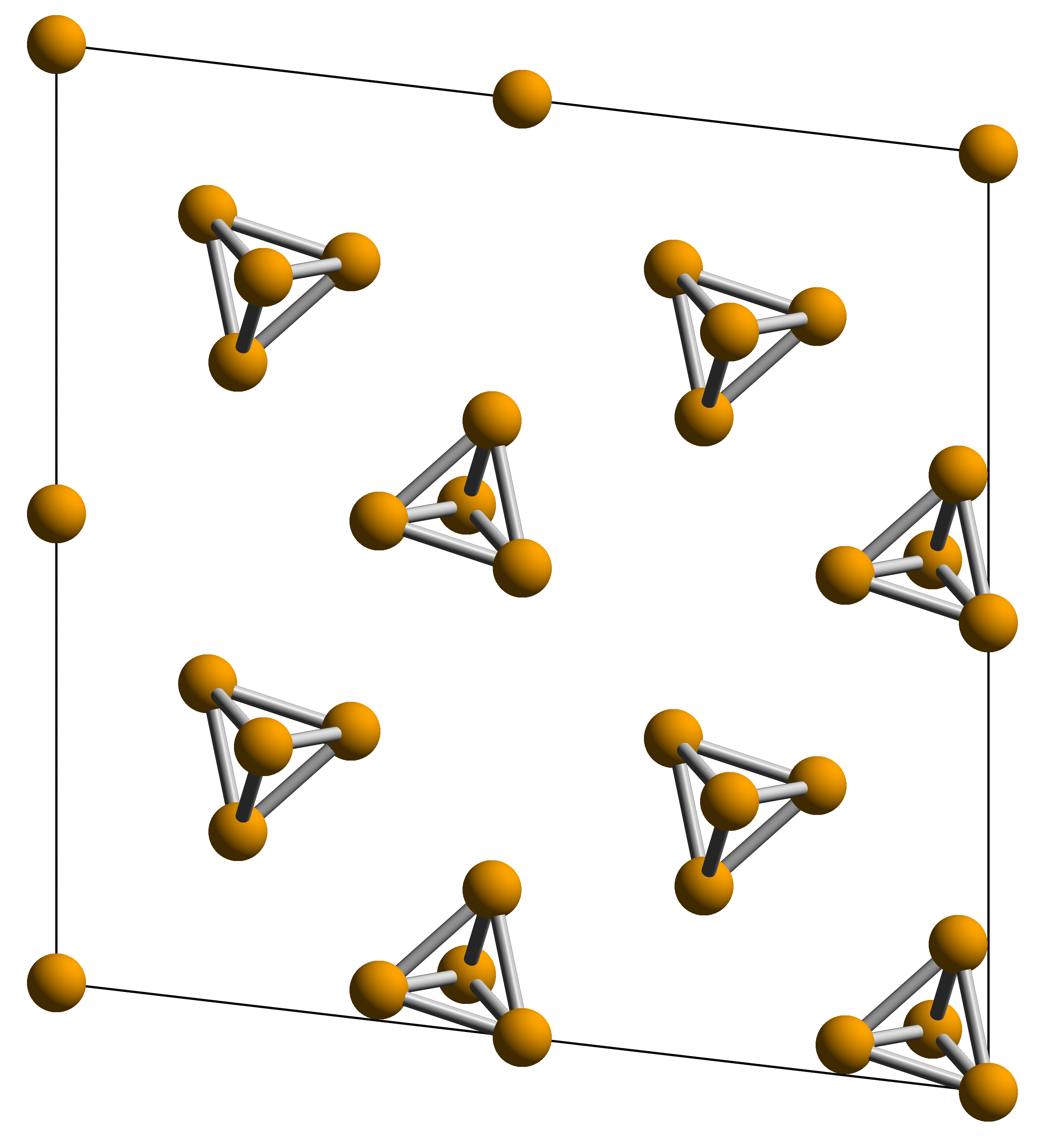|
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
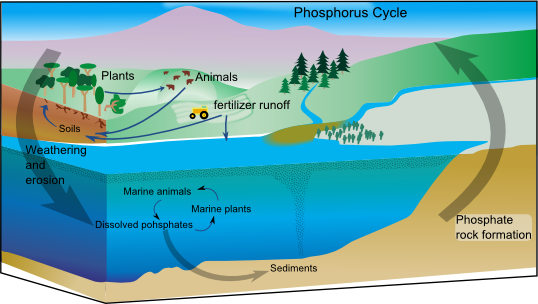
Phosphorus Cycle
The phosphorus cycle is the biogeochemical cycle that involves the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based materials do not enter the gaseous phase readily, as the main source of gaseous phosphorus, phosphine, is only produced in isolated and specific conditions. Therefore, the phosphorus cycle is primarily examined studying the movement of orthophosphate (), the form of phosphorus that is most commonly seen in the environment, through terrestrial and aquatic ecosystems. Living organisms require phosphorus, a vital component of DNA, RNA, ATP, etc., for their proper functioning. Phosphorus also enters in the composition of phospholipids present in cell membranes. Plants assimilate phosphorus as phosphate and incorporate it into organic compounds. In animals, inorganic phosphorus in the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pnictogen
, - ! colspan=2 style="text-align:left;" , ↓ Period , - ! 2 , , - ! 3 , , - ! 4 , , - ! 5 , , - ! 6 , , - ! 7 , , - , colspan="2", ---- ''Legend'' A pnictogen ( or ; from "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc). The IUPAC has called it Group 15 Since 1988. Before that, in America it was called Group VA, owing to a text by H. C. Deming and the Sargent-Welch Scientific Company, while in Europe it was called ecommended that in 1970. (Pronounced "group five A" and "group five B"; "V" is the Roman numeral 5). In semiconductor physics, it is still usually called Group V. The "five" ("V") in the historical names comes from the " pentavalency" of nitrogen, reflected by the stoichiometry of compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Red Phosphorus
Red phosphorus is an Allotropes of phosphorus, allotrope of phosphorus. It is an amorphous polymeric red solid that is stable in air. It can be easily converted from white phosphorus under light or heating. It finds applications as matches and fire retardants. It was discovered in 1847 by Anton von Schrötter. Structure Red phosphorus is an amorphous form of phosphorus. Crystalline forms of red phosphorus include Hittorf's phosphorus and fibrous red phosphorus. The structure of red phosphorus contains the fragments illustrated below: Preparation One method of preparing red phosphorus involves heating white phosphorus in an inert atmosphere like nitrogen or carbon dioxide, with iodine as catalyst. Another theoretically possible method of red phosphorus production is via light irradiation of white phosphorus. However, it has not been used industrially, likely due to the suspicious quality and unidentified structure the product. Properties Under standard conditions, red phosphor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphorus (morning Star)
Phosphorus () is the god of the planet Venus in its appearance as the Morning Star. Another Greek name for the Morning Star is "Eosphorus" (), which means "dawn-bringer". The term "eosphorus" is sometimes met in English. As an adjective, the word "phosphorus" is applied in the sense of "light-bringing" (for instance, the dawn, the god Dionysus, pine torches and the day) and "torch-bearing" as an epithet of several gods and goddesses, especially of Hecate but also of Artemis/ Diana and Hephaestus. Seasonally, Venus is the "light bringer" in the northern hemisphere, appearing most brightly in December (an optical illusion due to shorter days), signalling the "rebirth" of longer days as winter wanes. Venus The morning star is an appearance of the planet Venus, an inferior planet, meaning that its orbit lies between the Earth and the Sun. Depending on the orbital locations of both Venus and Earth, it can be seen in the eastern morning sky for an hour or so before the Sun rises ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
White Phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white " diphosphorus pentoxide", which consists of tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphate Rock
Phosphorite, phosphate rock or rock phosphate is a non- detrital sedimentary rock that contains high amounts of phosphate minerals. The phosphate content of phosphorite (or grade of phosphate rock) varies greatly, from 4% to 20% phosphorus pentoxide (P2O5). Marketed phosphate rock is enriched ("beneficiated") to at least 28%, often more than 30% P2O5. This occurs through washing, screening, de-liming, magnetic separation or flotation. By comparison, the average phosphorus content of sedimentary rocks is less than 0.2%.Blatt, Harvey and Robert J. Tracy, ''Petrology'', Freeman, 1996, 2nd ed. pp. 345–349 The phosphate is present as fluorapatite Ca5(PO4)3F typically in cryptocrystalline masses (grain sizes < 1 μm) referred to as collophane-sedimentary apatite deposits of uncertain origin. It is also present as |
Hennig Brand
Hennig Brand (; or ) was a German alchemist who lived and worked in Hamburg. In 1669, Brand accidentally discovered the chemical element phosphorus while searching for the "philosopher's stone", a substance which was believed to transmute base metals into gold. Biography The circumstances of Brand's birth are unknown but he was born in 1630 and died or 1710. Some sources describe his origins as humble and indicate that he had been an apprentice glassmaker as a young man. However, correspondence by his second wife Margaretha states that he was of high social standing. In any case he held a post as a junior army officer during the Thirty Years' War and his first wife's dowry was substantial, allowing him to pursue alchemy on leaving the army. He was one of the many searchers for the philosopher's stone. In the process, he accidentally discovered phosphorus. Discovery of phosphorus Like other alchemists of the time, Brand searched for the "philosopher's stone", a substance whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Match
A match is a tool for starting a fire. Typically, matches are made of small wooden sticks or stiff paper. One end is coated with a material that can be ignited by friction generated by striking the match against a suitable surface. Wooden matches are packaged in matchboxes, and paper matches are partially cut into rows and stapled into matchbooks. The coated end of a match, known as the match "head", consists of a bead of active ingredients and binder (material), binder, often colored for easier inspection. There are two main types of matches: safety matches, which can be struck only against a specially prepared surface, and strike-anywhere matches, for which any suitably frictional surface can be used. Etymology The word ''match'' derives from Old French ''mèche'', referring to the Candle wick, wick of a candle. Historically, the term ''match'' referred to lengths of rope, cord (later cambric) impregnated with chemicals, and allowed to burn continuously. These were used to li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and dry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fertiliser
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrition, plant nutrients. Fertilizers may be distinct from Liming (soil), liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and Agrochemical, industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods. Historically, fertilization came from natural or organic sources: compost, Manure, animal manure, Human waste, human manure, harvested minerals, crop rotations, and byproducts of human-nature industries (e.g. Fish meal, fish processing waste, or B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek language, Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (element), mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter Systematic element name, temporary sym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphate
Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, phosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one proton gives the dihydrogen phosphate ion while removal of two protons gives the hydrogen phosphate ion . These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate or orthophosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |