Phosphorus Cycle on:
[Wikipedia]
[Google]
[Amazon]
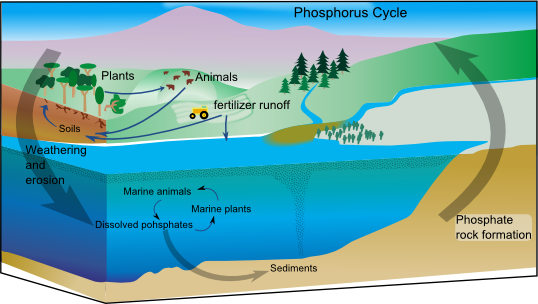 The phosphorus cycle is the
The phosphorus cycle is the


 Eutrophication is when waters are enriched by nutrients that lead to structural changes to the aquatic ecosystem such as algae bloom, deoxygenation, reduction of fish species. It does occur naturally, as when lakes age they become more productive due to increases in major limiting reagents such as nitrogen and phosphorus. For example, phosphorus can enter into lakes where it will accumulate in the sediments and the biosphere. It can also be recycled from the sediments and the water system allowing it to stay in the environment. Anthropogenic effects can also cause phosphorus to flow into aquatic ecosystems as seen in drainage water and runoff from fertilized soils on agricultural land. Additionally, eroded soils, which can be caused by deforestation and urbanization, can lead to more phosphorus and nitrogen being added to these aquatic ecosystems. These all increase the amount of phosphorus that enters the cycle which has led to excessive nutrient intake in freshwater systems causing dramatic growth in algal populations. When these algae die, their putrefaction depletes the water of oxygen and can toxify the waters. Both these effects cause plant and animal death rates to increase as the plants take in and animals drink the poisonous water.
Eutrophication is when waters are enriched by nutrients that lead to structural changes to the aquatic ecosystem such as algae bloom, deoxygenation, reduction of fish species. It does occur naturally, as when lakes age they become more productive due to increases in major limiting reagents such as nitrogen and phosphorus. For example, phosphorus can enter into lakes where it will accumulate in the sediments and the biosphere. It can also be recycled from the sediments and the water system allowing it to stay in the environment. Anthropogenic effects can also cause phosphorus to flow into aquatic ecosystems as seen in drainage water and runoff from fertilized soils on agricultural land. Additionally, eroded soils, which can be caused by deforestation and urbanization, can lead to more phosphorus and nitrogen being added to these aquatic ecosystems. These all increase the amount of phosphorus that enters the cycle which has led to excessive nutrient intake in freshwater systems causing dramatic growth in algal populations. When these algae die, their putrefaction depletes the water of oxygen and can toxify the waters. Both these effects cause plant and animal death rates to increase as the plants take in and animals drink the poisonous water.
 Oceanic ecosystems gather phosphorus through many sources, but it is mainly derived from weathering of rocks containing phosphorus which are then transported to the oceans in a dissolved form by river runoff. Due to a dramatic rise in mining for phosphorus, it is estimated that humans have increased the net storage of phosphorus in soil and ocean systems by 75%. This increase in phosphorus has led to more eutrophication in ocean waters as phytoplankton blooms have caused a drastic shift in anoxic conditions seen in both the
Oceanic ecosystems gather phosphorus through many sources, but it is mainly derived from weathering of rocks containing phosphorus which are then transported to the oceans in a dissolved form by river runoff. Due to a dramatic rise in mining for phosphorus, it is estimated that humans have increased the net storage of phosphorus in soil and ocean systems by 75%. This increase in phosphorus has led to more eutrophication in ocean waters as phytoplankton blooms have caused a drastic shift in anoxic conditions seen in both the
 The phosphorus cycle is the
The phosphorus cycle is the biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
that involves the movement of phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
through the lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
, hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
, and biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
. Unlike many other biogeochemical cycles, the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
does not play a significant role in the movement of phosphorus, because phosphorus and phosphorus-based materials do not enter the gaseous phase readily, as the main source of gaseous phosphorus, phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
, is only produced in isolated and specific conditions. Therefore, the phosphorus cycle is primarily examined studying the movement of orthophosphate (), the form of phosphorus that is most commonly seen in the environment, through terrestrial and aquatic ecosystems.
Living organisms require phosphorus, a vital component of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
, RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
, ATP, etc., for their proper functioning. Phosphorus also enters in the composition of phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s present in cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
s. Plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s assimilate phosphorus as phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
and incorporate it into organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s. In animal
Animals are multicellular, eukaryotic organisms in the Biology, biological Kingdom (biology), kingdom Animalia (). With few exceptions, animals heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, ...
s, inorganic phosphorus in the form of apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of Hydroxide, OH−, Fluoride, F− and Chloride, Cl− ion, respectively, in the crystal. The formula of the admixture of ...
() is also a key component of bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
s, teeth ( tooth enamel), etc. On the land, phosphorus gradually becomes less available to plants over thousands of years, since it is slowly lost in runoff. Low concentration of phosphorus in soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
s reduces plant growth and slows soil microbial growth, as shown in studies of soil microbial biomass
Biomass is a term used in several contexts: in the context of ecology it means living organisms, and in the context of bioenergy it means matter from recently living (but now dead) organisms. In the latter context, there are variations in how ...
. Soil microorganisms act as both sinks and sources of available phosphorus in the biogeochemical cycle. Short-term transformation of phosphorus is chemical, biological, or microbiological. In the long-term global cycle, however, the major transfer is driven by tectonic
Tectonics ( via Latin ) are the processes that result in the structure and properties of the Earth's crust and its evolution through time. The field of ''planetary tectonics'' extends the concept to other planets and moons.
These processes ...
movement over geologic time and weathering of phosphate containing rock such as apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of Hydroxide, OH−, Fluoride, F− and Chloride, Cl− ion, respectively, in the crystal. The formula of the admixture of ...
. Furthermore, phosphorus tends to be a limiting nutrient in aquatic ecosystem
An aquatic ecosystem is an ecosystem found in and around a body of water, in contrast to land-based terrestrial ecosystems. Aquatic ecosystems contain communities of organisms—aquatic life—that are dependent on each other and on their environ ...
s. However, as phosphorus enters aquatic ecosystems, it has the possibility to lead to over-production in the form of eutrophication
Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of organisms that may deplete the oxygen in the water; ie. the process of too many plants growing on the s ...
, which can happen in both freshwater and saltwater environments.
Human activities have caused major changes to the global phosphorus cycle primarily through the mining and subsequent transformation of phosphorus minerals for use in fertilizer and industrial products. Some phosphorus is also lost as effluent through the mining and industrial processes as well.
Phosphorus in the environment


Ecological function
Phosphorus is an essential nutrient for plants and animals. Phosphorus is a limiting nutrient for aquatic organisms. Phosphorus forms parts of important life-sustaining molecules that are very common in the biosphere. Phosphorus does enter the atmosphere in very small amounts when dust containing phosphorus is dissolved in rainwater and sea spray, but the element mainly remains on land and in rock and soil minerals. Phosphates which are found in fertilizers, sewage and detergents, can cause pollution in lakes and streams. Over-enrichment of phosphate in both fresh and inshore marine waters can lead to massive algae blooms. In fresh water, the death and decay of these blooms leads toeutrophication
Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of organisms that may deplete the oxygen in the water; ie. the process of too many plants growing on the s ...
. An example of this is the Canadian Experimental Lakes Area.
Freshwater algal blooms are generally caused by excess phosphorus, while those that take place in saltwater tend to occur when excess nitrogen is added. However, it is possible for eutrophication to be due to a spike in phosphorus content in both freshwater and saltwater environments.
Phosphorus occurs most abundantly in nature as part of the orthophosphate ion (PO4)3−, consisting of a P atom and 4 oxygen atoms. On land most phosphorus is found in rocks and minerals. Phosphorus-rich deposits have generally formed in the ocean or from guano, and over time, geologic processes bring ocean sediments to land. Weathering of rocks and minerals release phosphorus in a soluble form where it is taken up by plants, and it is transformed into organic compounds. The plants may then be consumed by herbivore
A herbivore is an animal anatomically and physiologically evolved to feed on plants, especially upon vascular tissues such as foliage, fruits or seeds, as the main component of its diet. These more broadly also encompass animals that eat ...
s and the phosphorus is either incorporated into their tissues or excreted. After death, the animal or plant decays, and phosphorus is returned to the soil where a large part of the phosphorus is transformed into insoluble compounds. Runoff may carry a small part of the phosphorus back to the ocean
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as ''oceans'' (the Pacific, Atlantic, Indian Ocean, Indian, Southern Ocean ...
. Generally with time (thousands of years) soils become deficient in phosphorus leading to ecosystem retrogression.
Major pools in aquatic systems
There are four major pools of phosphorus infreshwater
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. The term excludes seawater and brackish water, but it does include non-salty mi ...
ecosystems: dissolved inorganic phosphorus (DIP), dissolved organic phosphorus (DOP), particulate inorganic phosphorus (PIP) and particulate organic phosphorus (POP). Dissolved material is defined as substances that pass through a 0.45 μm filter. DIP consists mainly of orthophosphate () and polyphosphate, while DOP consists of DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and phosphoproteins. Particulate matter are the substances that get caught on a 0.45 μm filter and do not pass through. POP consists of both living and dead organisms, while PIP mainly consists of hydroxyapatite
Hydroxyapatite (International Mineralogical Association, IMA name: hydroxylapatite) (Hap, HAp, or HA) is a naturally occurring mineral form of calcium apatite with the Chemical formula, formula , often written to denote that the Crystal struc ...
, Ca5(PO4)3OH . Inorganic phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
comes in the form of readily soluble orthophosphate. Particulate organic phosphorus occurs in suspension in living and dead protoplasm and is insoluble. Dissolved organic phosphorus is derived from the particulate organic phosphorus by excretion and decomposition and is soluble.
Biological function
The primary biological importance of phosphates is as a component ofnucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s, which
serve as energy storage within cells ( ATP) or when linked together, form the nucleic acids DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
. The double helix of our DNA is only possible because of the phosphate ester bridge that binds the helix. Besides making biomolecules, phosphorus is also found in bone and the enamel of mammalian teeth, whose strength is derived from calcium phosphate in the form of hydroxyapatite
Hydroxyapatite (International Mineralogical Association, IMA name: hydroxylapatite) (Hap, HAp, or HA) is a naturally occurring mineral form of calcium apatite with the Chemical formula, formula , often written to denote that the Crystal struc ...
. It is also found in the exoskeleton of insects, and phospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s (found in all biological membranes). It also functions as a buffering agent in maintaining acid base homeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning fo ...
in the human body.
Phosphorus cycling
Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles. The global phosphorus cycle includes four major processes: :(i) tectonic uplift and exposure of phosphorus-bearing rocks such asapatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of Hydroxide, OH−, Fluoride, F− and Chloride, Cl− ion, respectively, in the crystal. The formula of the admixture of ...
to surface weathering;
:(ii) physical erosion, and chemical and biological weathering of phosphorus-bearing rocks to provide dissolved and particulate phosphorus to soils, lakes and rivers;
:(iii) riverine and subsurface transportation of phosphorus to various lakes and run-off to the ocean;
:(iv) sedimentation of particulate phosphorus (e.g., phosphorus associated with organic matter and oxide/carbonate minerals) and eventually burial in marine sediments (this process can also occur in lakes and rivers).
In terrestrial systems, bioavailable P (‘reactive P’) mainly comes from weathering of phosphorus-containing rocks. The most abundant primary phosphorus-mineral in the crust is apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of Hydroxide, OH−, Fluoride, F− and Chloride, Cl− ion, respectively, in the crystal. The formula of the admixture of ...
, which can be dissolved by natural acids generated by soil microbes and fungi, or by other chemical weathering reactions and physical erosion. The dissolved phosphorus is bioavailable to terrestrial organisms and plants and is returned to the soil after their decay. Phosphorus retention by soil minerals (e.g., adsorption onto iron and aluminum oxyhydroxides in acidic soils and precipitation onto calcite in neutral-to-calcareous soils) is usually viewed as the most important process in controlling terrestrial P-bioavailability in the mineral soil. This process can lead to the low level of dissolved phosphorus concentrations in soil solution. Various physiological strategies are used by plants and microorganisms for obtaining phosphorus from this low level of phosphorus concentration.
Soil phosphorus is usually transported to rivers and lakes and can then either be buried in lake sediments or transported to the ocean via river runoff. Atmospheric phosphorus deposition is another important marine phosphorus source to the ocean. In surface seawater, dissolved inorganic phosphorus, mainly orthophosphate (), is assimilated by phytoplankton and transformed into organic phosphorus compounds. Phytoplankton cell lysis releases cellular dissolved inorganic and organic phosphorus to the surrounding environment. Some of the organic phosphorus compounds can be hydrolyzed by enzymes synthesized by bacteria and phytoplankton and subsequently assimilated. The vast majority of phosphorus is remineralized within the water column, and approximately 1% of associated phosphorus carried to the deep sea by the falling particles is removed from the ocean reservoir by burial in sediments. A series of diagenetic processes act to enrich sediment pore water phosphorus concentrations, resulting in an appreciable benthic return flux of phosphorus to overlying bottom waters. These processes include
: (i) microbial respiration of organic matter in sediments,
: (ii) microbial reduction and dissolution of iron and manganese (oxyhydr)oxides with subsequent release of associated phosphorus, which connects the phosphorus cycle to the iron cycle
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient ...
, and
: (iii) abiotic reduction of iron (oxyhydr)oxides by hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
and liberation of iron-associated phosphorus.
Additionally,
: (iv) phosphate associated with calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
and
: (v) transformation of iron oxide-bound phosphorus to vivianite play critical roles in phosphorus burial in marine sediments.
These processes are similar to phosphorus cycling in lakes and rivers.
Although orthophosphate (), the dominant inorganic P species in nature, is oxidation state +5, certain microorganisms can use phosphonate and phosphite ( +3oxidation state) as a P source by oxidizing it to orthophosphate. Recently, rapid production and release of reduced phosphorus compounds has provided new clues about the role of reduced P as a missing link in oceanic phosphorus.
Phosphatic minerals
The availability of phosphorus in an ecosystem is restricted by its rate of release during weathering. The release of phosphorus fromapatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of Hydroxide, OH−, Fluoride, F− and Chloride, Cl− ion, respectively, in the crystal. The formula of the admixture of ...
dissolution is a key control on ecosystem productivity. The primary mineral with significant phosphorus content, apatite a5(PO4)3OHundergoes carbonation.
Little of this released phosphorus is taken up by biota, as it mainly reacts with other soil minerals. This leads to phosphorus becoming unavailable to organisms in the later stage of weathering and soil development as it will precipitate into rocks. Available phosphorus is found in a biogeochemical cycle in the upper soil profile, while phosphorus found at lower depths is primarily involved in geochemical reactions with secondary minerals. Plant growth depends on the rapid root uptake of phosphorus released from dead organic matter in the biochemical cycle. Phosphorus is limited in supply for plant growth. Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles.
Low-molecular-weight (LMW) organic acids are found in soils. They originate from the activities of various microorganisms in soils or may be exuded from the roots of living plants. Several of those organic acids are capable of forming stable organo-metal complexes with various metal ions found in soil solutions. As a result, these processes may lead to the release of inorganic phosphorus associated with aluminum, iron, and calcium in soil minerals. The production and release of oxalic acid by mycorrhizal fungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
explain their importance in maintaining and supplying phosphorus to plants.
The availability of organic phosphorus to support microbial, plant and animal growth depends on the rate of their degradation to generate free phosphate. There are various enzymes such as phosphatases, nucleases and phytase involved for the degradation. Some of the abiotic pathways in the environment studied are hydrolytic reactions and photolytic reactions. Enzymatic hydrolysis of organic phosphorus is an essential step in the biogeochemical phosphorus cycle, including the phosphorus nutrition of plants and microorganisms and the transfer of organic phosphorus from soil to bodies of water. Many organisms rely on the soil derived phosphorus for their phosphorus nutrition.
Dust Storms
P deposition is quite important for ecosystem function, and is unevenly distributed across the planet. Although phosphorus does not have a major atmospheric component, phosphorus sediments can be moved during dust storms. Large dust events can counteract natural imbalances where P occurs and allow for production in areas that would otherwise be P-limited. This component of the phosphorus cycle has generally been overlooked by researchers, but its importance is starting to be understood. Most of Earth's phosphorus is in rocks, and dust contains weathered rock particles from this parent material. Dust also carries other nutrients such as potassium, calcium, and magnesium, making these storms of high importance to biogeochemical cycling. The source of most large-scale dust storms are arid climates, including the Sahara Desert. Phosphorus carried in by wind from Northern Africa to the Amazon basin is thought to played a significant role supporting the rich biodiversity of the Amazon rainforest. These events are called dust-loading. Smaller scale dust-loading events have been found to occur in midwestern US, where erosion of agricultural land provides ideal conditions for dust storms. As the frequency of these dust storms increases, the amount of P left in the actual agricultural plots declines, leading to an increase of P fertilizer application. Retention of plant-available P becomes more difficult as erosion increases.Eutrophication
 Eutrophication is when waters are enriched by nutrients that lead to structural changes to the aquatic ecosystem such as algae bloom, deoxygenation, reduction of fish species. It does occur naturally, as when lakes age they become more productive due to increases in major limiting reagents such as nitrogen and phosphorus. For example, phosphorus can enter into lakes where it will accumulate in the sediments and the biosphere. It can also be recycled from the sediments and the water system allowing it to stay in the environment. Anthropogenic effects can also cause phosphorus to flow into aquatic ecosystems as seen in drainage water and runoff from fertilized soils on agricultural land. Additionally, eroded soils, which can be caused by deforestation and urbanization, can lead to more phosphorus and nitrogen being added to these aquatic ecosystems. These all increase the amount of phosphorus that enters the cycle which has led to excessive nutrient intake in freshwater systems causing dramatic growth in algal populations. When these algae die, their putrefaction depletes the water of oxygen and can toxify the waters. Both these effects cause plant and animal death rates to increase as the plants take in and animals drink the poisonous water.
Eutrophication is when waters are enriched by nutrients that lead to structural changes to the aquatic ecosystem such as algae bloom, deoxygenation, reduction of fish species. It does occur naturally, as when lakes age they become more productive due to increases in major limiting reagents such as nitrogen and phosphorus. For example, phosphorus can enter into lakes where it will accumulate in the sediments and the biosphere. It can also be recycled from the sediments and the water system allowing it to stay in the environment. Anthropogenic effects can also cause phosphorus to flow into aquatic ecosystems as seen in drainage water and runoff from fertilized soils on agricultural land. Additionally, eroded soils, which can be caused by deforestation and urbanization, can lead to more phosphorus and nitrogen being added to these aquatic ecosystems. These all increase the amount of phosphorus that enters the cycle which has led to excessive nutrient intake in freshwater systems causing dramatic growth in algal populations. When these algae die, their putrefaction depletes the water of oxygen and can toxify the waters. Both these effects cause plant and animal death rates to increase as the plants take in and animals drink the poisonous water.
Saltwater phosphorus eutrophication
 Oceanic ecosystems gather phosphorus through many sources, but it is mainly derived from weathering of rocks containing phosphorus which are then transported to the oceans in a dissolved form by river runoff. Due to a dramatic rise in mining for phosphorus, it is estimated that humans have increased the net storage of phosphorus in soil and ocean systems by 75%. This increase in phosphorus has led to more eutrophication in ocean waters as phytoplankton blooms have caused a drastic shift in anoxic conditions seen in both the
Oceanic ecosystems gather phosphorus through many sources, but it is mainly derived from weathering of rocks containing phosphorus which are then transported to the oceans in a dissolved form by river runoff. Due to a dramatic rise in mining for phosphorus, it is estimated that humans have increased the net storage of phosphorus in soil and ocean systems by 75%. This increase in phosphorus has led to more eutrophication in ocean waters as phytoplankton blooms have caused a drastic shift in anoxic conditions seen in both the Gulf of Mexico
The Gulf of Mexico () is an oceanic basin and a marginal sea of the Atlantic Ocean, mostly surrounded by the North American continent. It is bounded on the northeast, north, and northwest by the Gulf Coast of the United States; on the southw ...
and the Baltic Sea
The Baltic Sea is an arm of the Atlantic Ocean that is enclosed by the countries of Denmark, Estonia, Finland, Germany, Latvia, Lithuania, Poland, Russia, Sweden, and the North European Plain, North and Central European Plain regions. It is the ...
. Some research suggests that when anoxic conditions arise from eutrophication due to excess phosphorus, this creates a positive feedback loop that releases more phosphorus from oceanic reserves, exacerbating the issue. This could possibly create a self-sustaining cycle of oceanic anoxia where the constant recovery of phosphorus keeps stabilizing the eutrophic growth. Attempts to mitigate this problem using biological approaches are being investigated. One such approach involves using phosphorus accumulating organisms such as, '' Candidatus accumulibacter phosphatis'', which are capable of effectively storing phosphorus in the form of phosphate in marine ecosystems. Essentially, this would alter how the phosphorus cycle exists currently in marine ecosystems. Currently, there has been a major influx of phosphorus due to increased agricultural use and other industrial applications, thus these organisms could theoretically store phosphorus and hold on to it until it could be recycled in terrestrial ecosystems which would have lost this excess phosphorus due to runoff.
Wetland
Wetlands are frequently applied to solve the issue of eutrophication. Nitrate is transformed in wetlands to free nitrogen and discharged to the air. Phosphorus is adsorbed by wetland soils which are taken up by the plants. Therefore, wetlands could help to reduce the concentration of nitrogen and phosphorus to remit eutrophication. However, wetland soils can only hold a limited amount of phosphorus. To remove phosphorus continually, it is necessary to add more new soils within the wetland from remnant plant stems, leaves, root debris, and undecomposable parts of dead algae, bacteria, fungi, and invertebrates.Interaction with Nitrogen
Both N and P are widely used in agricultural fertilizers, as they are essential nutrients for plants. Human activity has resulted in an imbalance of normal N:P ratios, impacting the speed at which organisms synthesize proteins and DNA. In the last 40 years, the N:P ratio has increased from 19:1 to 30:1, meaning P is less available to ecosystems. This imbalance is not only caused by more N pollution, but also because P is more likely to get trapped after water has been treated, preventing its release into ecosystems. In an environment where neither nutrient is limited, where more P is present per N, organisms experience a faster growth rate. As this ratio increases, it is harder for organisms to grow. One example of organism response to this growing imbalance is rhizobia in legume root nodules. Research has shown that in low levels of P, the capacity for nitrogen-fixing bacteria to provide nutrients to the plant declines, negatively impacting both the host plant and its symbionts. In addition, plants growing in P-limited environments will have more N content in their leaves. P and N are also unequally distributed across the globe, making certain geographical areas more favorable for crop growth than others. Disruption to these major biogeochemical cycles may exacerbate these inequities.Human influences
Nutrients are important to the growth and survival of living organisms and, hence, are essential for developing and maintaining healthy ecosystems. Humans have greatly influenced the phosphorus cycle by mining phosphate rock. For millennia, phosphorus was primarily brought into the environment by weathering phosphate-containing rocks, which would replenish the phosphorus normally lost to the environment through processes such as runoff, albeit on a very slow and gradual time scale. Since the 1840s, when the technology to mine and extract phosphorus became more prevalent, approximately 110 teragrams of phosphorus has been added to the environment. This trend appears to be continuing in the future as from 1900-2022, the amount of phosphorus mined globally has increased 72-fold, with an expected annual increase of 4%. Most of this mining is done to produce fertilizers which can be used on a global scale. However, at the rate humans are mining, the geological system can not quickly restore what is lost. Thus, researchers are examining ways to better recycle phosphorus in the environment, with one promising application including the use of microorganisms. Regardless, humans have had a profound impact on the phosphorus cycle with wide-reaching implications aboutfood security
Food security is the state of having reliable access to a sufficient quantity of affordable, healthy Human food, food. The availability of food for people of any class, gender, ethnicity, or religion is another element of food protection. Simila ...
, eutrophication
Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of organisms that may deplete the oxygen in the water; ie. the process of too many plants growing on the s ...
, and the overall availability of the nutrient.
Other human processes can have detrimental effects on the phosphorus cycle, such as the repeated application of liquid hog manure in excess to crops. Applying biosolids may also increase available phosphorus in soil. In poorly drained soils or in areas where snowmelt can cause periodic waterlogging, reducing conditions can be attained in 7–10 days. This causes a sharp increase in phosphorus concentration in solution, and phosphorus can be leached. In addition, reducing the soil causes a shift in phosphorus from resilient to more labile forms. This could eventually increase the potential for phosphorus loss. This is of particular concern for the environmentally sound management of such areas, where disposal of agricultural wastes has already become a problem. It is suggested that soil water regimes used for organic waste disposal be considered when preparing waste management regulations.
See also
* Planetary boundaries * Oceanic carbon cycleReferences
External links
* * * * * {{Authority control Biogeochemical cycle Soil biology Soil chemistry Phosphorus