|
Phase Separation
Phase separation is the creation of two distinct Phase (matter), phases from a single homogeneous mixture. The most common type of phase separation is between two immiscible liquids, such as oil and water. This type of phase separation is known as liquid-liquid equilibrium. Colloids are formed by phase separation, though not all phase separations forms colloids - for example oil and water can form separated layers under gravity rather than remaining as microscopic droplets in suspension. A common form of spontaneous phase separation is termed spinodal decomposition; it is described by the Cahn–Hilliard equation. Regions of a phase diagram in which phase separation occurs are called miscibility gaps. There are two boundary curves of note: the binodal, binodal coexistence curve and the spinodal, spinodal curve. On one side of the binodal, mixtures are absolutely stable. In between the binodal and the spinodal, mixtures may be metastable: staying mixed (or unmixed) absent some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Separation Janus Particles
Phase or phases may refer to: Science *State of matter, or phase, one of the distinct forms in which matter can exist *Phase (matter), a region of space throughout which all physical properties are essentially uniform *Phase space, a mathematical space in which each possible state of a physical system is represented by a point also referred to as a "microscopic state" **Phase space formulation, a formulation of quantum mechanics in phase space *Phase (waves), the position of a point in time (an instant) on a waveform cycle **Instantaneous phase, generalization for both cyclic and non-cyclic phenomena *AC phase, the phase offset between alternating current electric power in multiple conducting wires **Single-phase electric power, distribution of AC electric power in a system where the voltages of the supply vary in unison **Three-phase electric power, a common method of AC electric power generation, transmission, and distribution *Phase problem, the loss of information (the phase) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
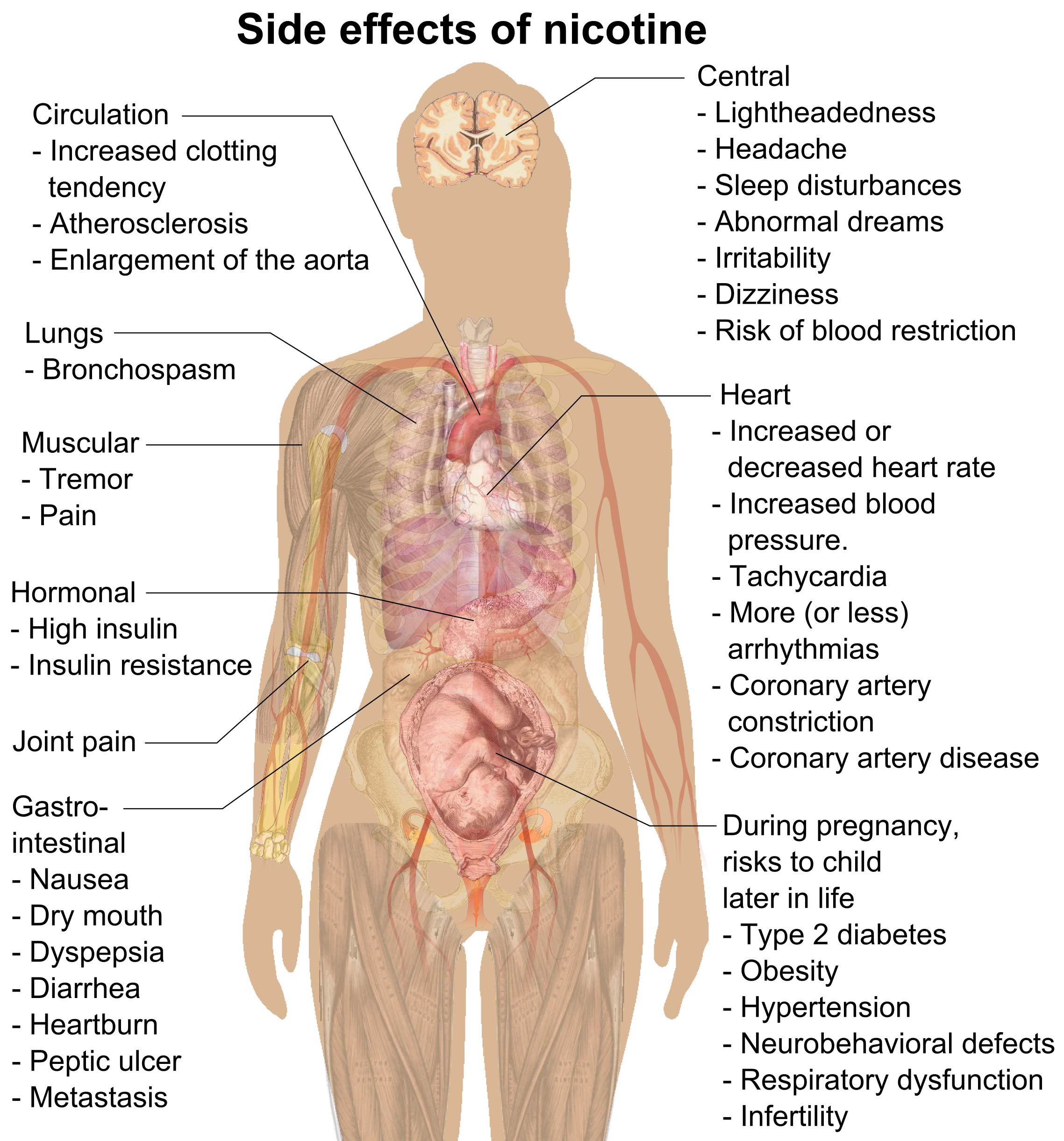
Nicotine
Nicotine is a natural product, naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreational drug use, recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve drug withdrawal, withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as a receptor antagonist. Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco. Nicotine is also present at Parts-per notation, ppb concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants, though sources disagree on whether this has any biological significance to human consumers. It functions as an plant defense against herbivory, antiherbivore toxin; consequently, nicotine was widely used as an insecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Vortex
In physics, a quantum vortex represents a quantized flux circulation of some physical quantity. In most cases, quantum vortices are a type of topological defect exhibited in superfluids and superconductors. The existence of quantum vortices was first predicted by Lars Onsager in 1949 in connection with superfluid helium. Onsager reasoned that quantisation of vorticity is a direct consequence of the existence of a superfluid order parameter as a spatially continuous wavefunction. Onsager also pointed out that quantum vortices describe the circulation of superfluid and conjectured that their excitations are responsible for superfluid phase transitions. These ideas of Onsager were further developed by Richard Feynman in 1955 and in 1957 were applied to describe the magnetic phase diagram of type-II superconductors by Alexei Alexeyevich Abrikosov. In 1935 Fritz London published a very closely related work on magnetic flux quantization in superconductors. London's fluxoid can also be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Gas
A Fermi gas is an idealized model, an ensemble of many non-interacting fermions. Fermions are particles that obey Fermi–Dirac statistics, like electrons, protons, and neutrons, and, in general, particles with half-integer spin. These statistics determine the energy distribution of fermions in a Fermi gas in thermal equilibrium, and is characterized by their number density, temperature, and the set of available energy states. The model is named after the Italian physicist Enrico Fermi. This physical model is useful for certain systems with many fermions. Some key examples are the behaviour of charge carriers in a metal, nucleons in an atomic nucleus, neutrons in a neutron star, and electrons in a white dwarf. Description An ideal Fermi gas or free Fermi gas is a physical model assuming a collection of non-interacting fermions in a constant potential well. Fermions are elementary or composite particles with half-integer spin, thus follow Fermi–Dirac statistics. The e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultracold Atom
In condensed matter physics, an ultracold atom is an atom with a temperature near absolute zero. At such temperatures, an atom's quantum-mechanical properties become important, especially through what's known as a "superfluid", such as Superfluid Helium 4. To reach such low temperatures, a combination of several techniques typically has to be used. First, atoms are trapped and pre-cooled via laser cooling in a magneto-optical trap. To reach the lowest possible temperature, further cooling is performed using evaporative cooling in a magnetic or optical trap. Several Nobel prizes in physics are related to the development of the techniques to manipulate quantum properties of individual atoms (e.g. 1989, 1996, 1997, 2001, 2005, 2012, 2018). Experiments with ultracold atoms study a variety of phenomena, including quantum phase transitions, Bose–Einstein condensation (BEC), bosonic superfluidity, quantum magnetism, many-body spin dynamics, Efimov states, Bardeen–Cooper� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Helium-4 makes up about one quarter of the ordinary matter in the universe by mass, with almost all of the rest being hydrogen. While nuclear fusion in stars also produces helium-4, most of the helium-4 in the Sun and in the universe is thought to have been produced during the Big Bang, known as " primordial helium". However, primordial helium-4 is largely absent from the Earth, having escaped during the high-temperature phase of Earth's formation. On Earth, most naturally occurring helium-4 is produced by the alpha decay of heavy elements in the Earth's crust, after the planet cooled and solidified. When liquid helium-4 is cooled to below , it becomes a superfluid, with properties very different from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with more protons than neutrons. It was discovered in 1939. Helium-3 atoms are fermionic and become a superfluid at the temperature of 2.491 mK. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. It is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some found in the terrestrial atmosphere is a remnant of atmospheric and underwater nuclear weapons testing. Nuclear fusion using helium-3 has long been viewed as a desirable future energy source. The fusion of two of its atoms would be aneut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Mixture
An ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing. The vapor pressures of all components obey Raoult's law across the entire range of concentrations, and the activity coefficient (which measures deviation from ideality) is equal to one for each component. The concept of an ideal solution is fundamental to both thermodynamics and chemical thermodynamics and their applications, such as the explanation of colligative properties. Physical origin Ideality of solutions is analogous to ideality for gases, with the important difference that intermolecular interactions in liquids are strong and cannot simply be neglected as they can for ideal gases. Instead we assume that the mean strength of the interactions are the same between all the molecules of the solution. More formally, for a mix of molecules of A and B, then the interaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Of Mixing
In thermodynamics, the entropy of mixing is the increase in the total entropy when several initially separate systems of different composition, each in a thermodynamic state of internal equilibrium, are mixed without chemical reaction by the thermodynamic operation of removal of impermeable partition(s) between them, followed by a time for establishment of a new thermodynamic state of internal equilibrium in the new unpartitioned closed system. In general, the mixing may be constrained to occur under various prescribed conditions. In the customarily prescribed conditions, the materials are each initially at a common temperature and pressure, and the new system may change its volume, while being maintained at that same constant temperature, pressure, and chemical component masses. The volume available for each material to explore is increased, from that of its initially separate compartment, to the total common final volume. The final volume need not be the sum of the initially se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Mixing
In thermodynamics, the enthalpy of mixing (also heat of mixing and excess enthalpy) is the enthalpy liberated or absorbed from a substance upon mixing. When a substance or compound is combined with any other substance or compound, the enthalpy of mixing is the consequence of the new interactions between the two substances or compounds. This enthalpy, if released exothermically, can in an extreme case cause an explosion. Enthalpy of mixing can often be ignored in calculations for mixtures where other heat terms exist, or in cases where the mixture is ideal. The sign convention is the same as for enthalpy of reaction: when the enthalpy of mixing is positive, mixing is endothermic, while negative enthalpy of mixing signifies exothermic mixing. In ideal mixtures, the enthalpy of mixing is null. In non-ideal mixtures, the thermodynamic activity of each component is different from its concentration by multiplying with the activity coefficient. One approximation for calculating t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change and information systems including the transmission of information in telecommunication. Entropy is central to the second law of thermodynamics, which states that the entropy of an isolated system left to spontaneous evolution cannot decrease with time. As a result, isolated systems evolve toward thermodynamic equilibrium, where the entropy is highest. A consequence of the second law of thermodynamics is that certain processes are irreversible. The thermodynami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |