|
Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Helium-4 makes up about one quarter of the ordinary matter in the universe by mass, with almost all of the rest being hydrogen. While nuclear fusion in stars also produces helium-4, most of the helium-4 in the Sun and in the universe is thought to have been produced during the Big Bang, known as " primordial helium". However, primordial helium-4 is largely absent from the Earth, having escaped during the high-temperature phase of Earth's formation. On Earth, most naturally occurring helium-4 is produced by the alpha decay of heavy elements in the Earth's crust, after the planet cooled and solidified. When liquid helium-4 is cooled to below , it becomes a superfluid, with properties very different from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium Atom QM
Helium (from ) is a chemical element; it has symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, the vast majority of which was formed during the Big Bang. Large amounts of n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Big Bang Nucleosynthesis
In physical cosmology, Big Bang nucleosynthesis (also known as primordial nucleosynthesis, and abbreviated as BBN) is a model for the production of light nuclei, deuterium, 3He, 4He, 7Li, between 0.01s and 200s in the lifetime of the universe. The model uses a combination of thermodynamic arguments and results from equations for the expansion of the universe to define a changing temperature and density, then analyzes the rates of nuclear reactions at these temperatures and densities to predict the nuclear abundance ratios. Refined models agree very well with observations with the exception of the abundance of 7Li. The model is one of the key concepts in standard cosmology. Elements heavier than lithium are thought to have been created later in the life of the Universe by stellar nucleosynthesis, through the formation, evolution and death of stars. Characteristics The Big Bang nucleosynthesis (BBN) model assumes a homogeneous plasma, at a temperature corresponding to 1 MeV, co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superfluid Helium-4
Superfluid helium-4 (helium II or He-II) is the superfluid form of helium-4, the most common isotope of the element helium. The substance, which resembles other liquids such as helium I (conventional, non-superfluid liquid helium), flows without friction past any surface, which allows it to continue to circulate over obstructions and through pores in containers which hold it, subject only to its own inertia. The formation of the superfluid is a manifestation of the formation of a Bose–Einstein condensate of helium atoms. This condensation occurs in liquid helium-4 at a far higher temperature (2.17 K) than it does in helium-3 (2.5 mK) because each atom of helium-4 is a boson particle, by virtue of its zero Spin (physics), spin. Helium-3, however, is a fermion particle, which can form bosons only by pairing with itself at much lower temperatures, in a weaker process that is similar to the electron pairing in superconductivity. History Known as a major facet in the stu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Star
A star is a luminous spheroid of plasma (physics), plasma held together by Self-gravitation, self-gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sky, night; their immense distances from Earth make them appear as fixed stars, fixed points of light. The most prominent stars have been categorised into constellations and asterism (astronomy), asterisms, and many of the brightest stars have proper names. Astronomers have assembled star catalogues that identify the known stars and provide standardized stellar designations. The observable universe contains an estimated to stars. Only about 4,000 of these stars are visible to the naked eye—all within the Milky Way galaxy. A star's life star formation, begins with the gravitational collapse of a gaseous nebula of material largely comprising hydrogen, helium, and traces of heavier elements. Its stellar mass, total mass mainly determines it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay. When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic element, monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the release or absorption (electromagnetic radiation), absorption of energy. This difference in mass arises as a result of the difference in nuclear binding energy between the atomic nuclei before and after the fusion reaction. Nuclear fusion is the process that powers all active stars, via many Stellar nucleosynthesis, reaction pathways. Fusion processes require an extremely large Lawson criterion, triple product of temperature, density, and confinement time. These conditions occur only in Stellar core, stellar cores, advanced Nuclear weapon design, nuclear weapons, and are approached in List of fusion experiments, fusion power experiments. A nuclear fusion process that produces atomic nuclei lighter than nickel-62 is generally exothermic, due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
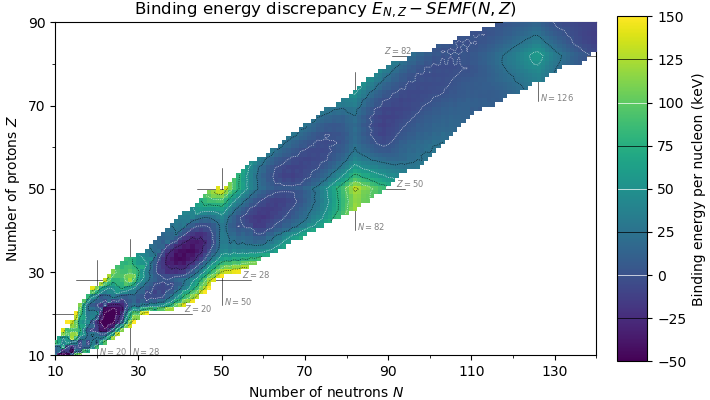
Magic Number (physics)
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a "magic" number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126. For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead, and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay. The unusual stability of isotopes having magic numbers means that transuranium elements could theoretically be created with extremely large nuclei and yet not be subject ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of ''ħ'', but with a much greater mass. It is classified as a lepton. As with other leptons, the muon is not thought to be composed of any simpler particles. The muon is an unstable subatomic particle with a mean lifetime of , much longer than many other subatomic particles. As with the decay of the free neutron (with a lifetime around 15 minutes), muon decay is slow (by subatomic standards) because the decay is mediated only by the weak interaction (rather than the more powerful strong interaction or electromagnetic interaction), and because the mass difference between the muon and the set of its decay products is small, providing few kinetic degrees of freedom for decay. Muon decay almost always produces at least three particles, which must include an electron of the same charge as the muon and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymously with amorphous solid; however, these terms refer specifically to amorphous materials that undergo a glass transition. Examples of amorphous solids include glasses, metallic glasses, and certain types of plastics and polymers. Etymology The term "Amorphous" comes from the Greek language, Greek ''a'' ("without"), and ''morphé'' ("shape, form"). Structure Amorphous materials have an internal structure of molecular-scale structural blocks that can be similar to the basic structural units in the crystalline phase of the same compound. Unlike in crystalline materials, however, no long-range regularity exists: amorphous materials cannot be described by the repetition of a finite unit cell. Statistical measures, such as the atomic density ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Cloud
In quantum mechanics, an atomic orbital () is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calculate the probability of finding an electron in a specific region around the nucleus. Each orbital in an atom is characterized by a set of values of three quantum numbers , , and , which respectively correspond to electron's energy, its orbital angular momentum, and its orbital angular momentum projected along a chosen axis ( magnetic quantum number). The orbitals with a well-defined magnetic quantum number are generally complex-valued. Real-valued orbitals can be formed as linear combinations of and orbitals, and are often labeled using associated harmonic polynomials (e.g., ''xy'', ) which describe their angular structure. An orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Femtometer
The femtometre (American spelling femtometer), symbol fm, (derived from the Danish and Norwegian word 'fifteen', ) is a unit of length in the International System of Units (SI) equal to 10−15 metres, which means a quadrillionth of one metre. This distance is sometimes called a fermi and was so named in honour of Italian naturalized to American physicist Enrico Fermi, as it is a typical length-scale of nuclear physics. Definition and equivalents 1000000 zeptometres = 1 femtometre = 1 fermi = 0.000001 nanometre = femtometres = 1 millimetre. For example, the charge radius of a proton is approximately 0.841 femtometres while the radius of a gold nucleus is approximately 8.45 femtometres. 1 barn = 100 fm2 History The femtometre was adopted by the 11th ''Conférence Générale des Poids et Mesures'', and added to the SI in 1964, using the Danish word for "15" and the similarity in spelling with ''fermi''. The fermi is named after the Itali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |