|
Pentamethylantimony
Pentamethylantimony or pentamethylstiborane is an organometalllic compound containing five methyl groups bound to an antimony atom with formula Sb(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Some other antimony(V) organometallic compounds include pentapropynylantimony (Sb(CCCH3)5) and pentaphenyl antimony (Sb(C6H5)5). Other known pentamethyl-pnictides include pentamethylbismuth and pentamethylarsenic. Production Pentamethylantimony can be made by reacting Sb(CH3)3Br2 with two equivalents of methyl lithium. Another production route is to convert trimethylstibine to the trimethyl antimony dichloride, and then replace the chlorine with methyl groups with methyl lithium. :Sb(CH3)3 + Cl2 → Sb(CH3)3Cl2 :Sb(CH3)3Cl2 + 2LiCH3 → Sb(CH3)5 + 2LiCl Properties Pentamethylantimony is colourless. At -143 °C it crystallizes in the orthorhombic system with space group ''Ccmm''. Unit cell dimensions are a=6.630 Å b=11.004&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylarsenic
Pentamethylarsenic (or pentamethylarsorane) is an organometalllic compound containing five methyl groups bound to an arsenic atom with formula As(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. History The first claim to make pentamethylarsenic was in 1862 in a reaction of tetramethylarsonium iodide with dimethylzinc by A. Cahours. For many years all the reproductions of this proved fruitless, so the production proved not to be genuine. It was actually discovered by Karl-Heinz Mitschke and Hubert Schmidbaur in 1973. Production Trimethylarsine is chlorinated to trimethylarsine dichloride, which then reacts with methyl lithium to yield pentamethylarsenic. :As(CH3)3 + Cl2 → As(CH3)3Cl2 :As(CH3)3Cl2 + 2LiCH3 → As(CH3)5 + 2LiCl Side products include As(CH3)4Cl and As(CH3)3=CH2. Pentamethylarsenic is not produced by biological organisms. Properties Pentamethylarsenic smells the same as pentamethylantimony, but is otherwise unique. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylstibine
Trimethylstibine is an organoantimony compound with the formula Sb(CH3)3. It is a colorless pyrophoric and toxic liquid. It is synthesized by treatment of antimony trichloride and methyl Grignard reagent.Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf "Antimony and Antimony Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. It is produced by anaerobic bacteria in antimony-rich soils. In contrast to trimethylphosphine, trimethylstibine is a weaker Lewis base. It is used in the production of some III-V semiconductor Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of ...s. References Organoantimony compounds Methyl compounds {{alkanederivative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluorophosphoric Acid
Difluorophosphoric acid is an inorganic compound with the formula . It is a mobile colorless strongly fuming liquid. The acid has limited applications, in part because it is thermally and hydrolytically unstable. Difluorophosphoric acid is corrosive to glass, fabric, metals and living tissue. A method to make pure difluorphosphoric acid involves heating phosphoryl fluoride with fluorophosphoric acid and separating the product by distillation: : It is prepared by hydrolysis of phosphoryl fluoride: : Further hydrolysis gives fluorophosphoric acid: : Complete hydrolysis gives phosphoric acid Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...: : The salts of difluorophosphoric acid are known as difluorophosphates. References {{inorganic-compound-stub Oxohalides Phosphorus ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazoic Acid
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes. Hydrazoic acid, like its fellow mineral acids, is soluble in water. Undiluted hydrazoic acid is dangerously explosive with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJ/mol. When dilute, the gas and aqueous solutions (<10%) can be safely prepared but should be used immediately; because of its low boiling point, hydrazoic acid is enriched upon evaporation and condensation such that dilute solutions incapable of explosion can form droplets in the headspace of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocyanic Acid
Thiocyanic acid is a chemical compound with the formula and structure , which exists as a tautomer with isothiocyanic acid (). The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase. : It is a moderately strong acid, with a p''K''a of 1.1 at 20 °C and extrapolated to zero ionic strength. One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen. Thiocyanic acid has been observed spectroscopically. The salts and esters of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion () and a suitable cation (e.g., potassium thiocyanate Potassium thiocyanate is the chemical compound with the molecular formula KSCN. It is an important salt of the thiocyanate anion, one of the pseudohalides. The compound has a low melting point relative to most other inorganic salts. Uses Ch ..., KSCN). The esters of thiocyanic acid h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
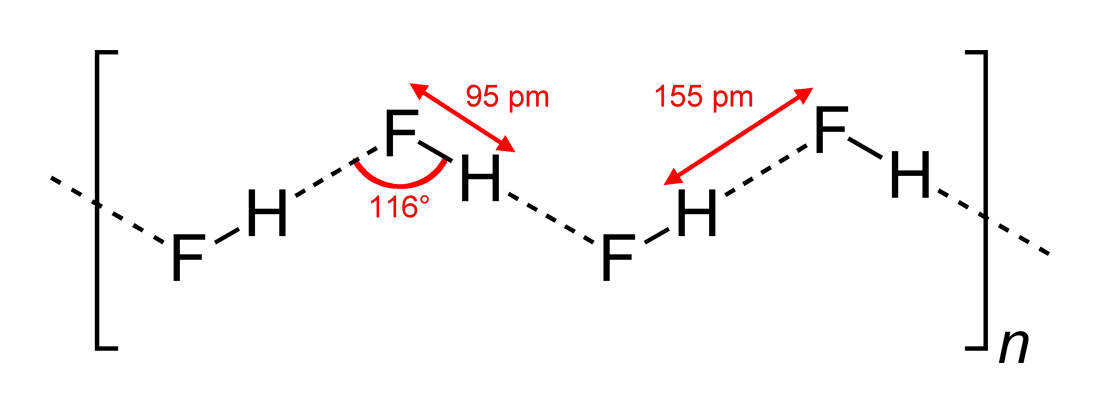
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannocene
Stannocene is an organometallic compound with the formula . It appears yellow-brown, and melts around 105 °C. Chemically, stannocene is a metallocene that can be produced efficiently from cyclopentadienyl sodium and tin(II) chloride. Unlike in ferrocene the two cyclopentadienyl Cyclopentadienyl can refer to * Cyclopentadienyl anion, or cyclopentadienide, ** Cyclopentadienyl ligand * Cyclopentadienyl radical, • * Cyclopentadienyl cation, See also * Pentadienyl {{Chemistry index ... rings are not parallel. References Organotin compounds Metallocenes Tin(II) compounds Cyclopentadienyl complexes {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |