|
PSEPT
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade, and were further developed by others including Michael Mingos; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These notes contained original material that served as the basis of the sections on the 4''n'', 5''n'', and 6''n'' rules. These rules have been extended and unified in the form of the Jemmis ''mno'' rules. Predicting structures of cluster compounds Different rules (4''n'', 5''n'', or 6''n'') are invoked depending on the number of electrons per vertex. The 4''n'' rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case for many boranes and carboranes. For such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
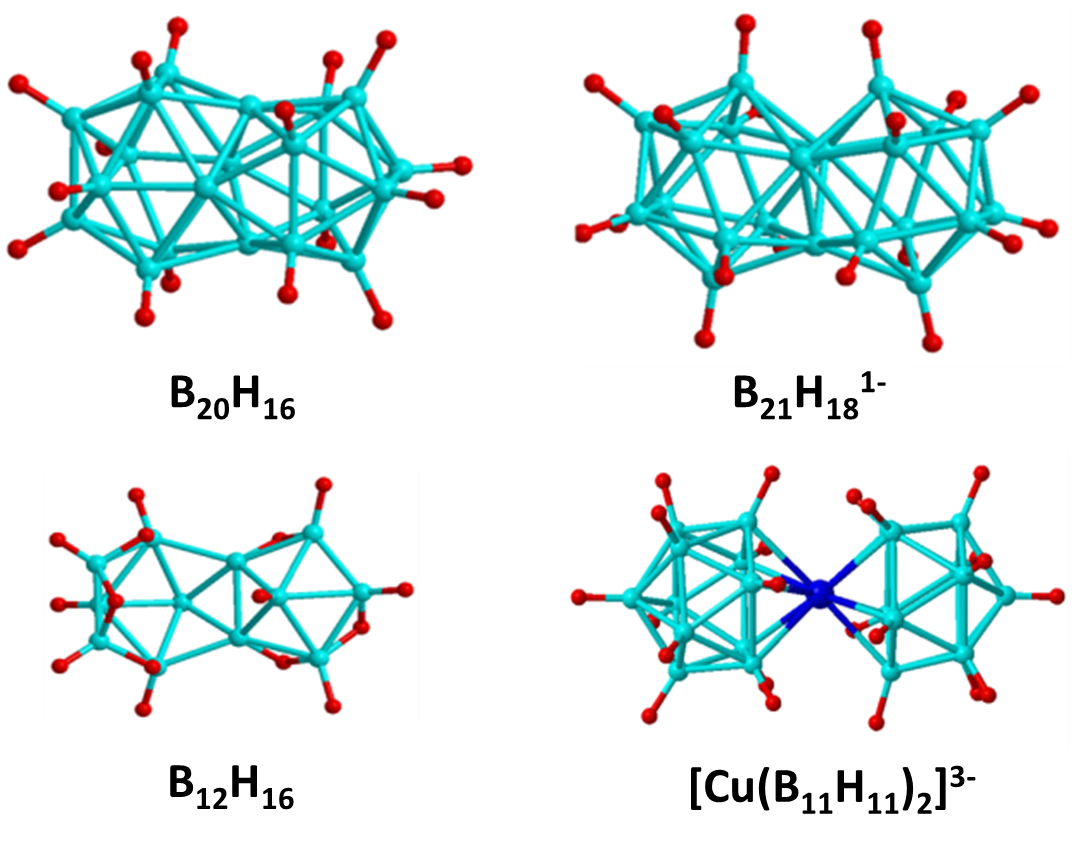
Jemmis Mno Rules
In chemistry, the Jemmis ''mno'' rules represent a unified rule for predicting and systematizing structures of compounds, usually clusters. The rules involve electron counting. They were formulated by E. D. Jemmis to explain the structures of condensed polyhedral boranes such as , which are obtained by condensing polyhedral boranes by sharing a triangular face, an edge, a single vertex, or four vertices. These rules are additions and extensions to Wade's rules and polyhedral skeletal electron pair theory. The Jemmis ''mno'' rule provides the relationship between polyhedral boranes, condensed polyhedral boranes, and β-rhombohedral boron. This is similar to the relationship between benzene, condensed benzenoid aromatics, and graphite, shown by Hückel's 4''n'' + 2 rule, as well as the relationship between tetracoordinate tetrahedral carbon compounds and diamond. The Jemmis ''mno'' rules reduce to Hückel's rule when restricted to two dimensions and reduce to Wade's rul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tetrahedron is the simplest of all the ordinary convex polytope, convex polyhedra. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean geometry, Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid (geometry), pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron, the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such net (polyhedron), nets. For any tetrahedron there exists a sphere (called th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S4 Cluster
S4, S 4, Š-4, S.4 or S-4 may refer to: People * S4 (gamer), Gustav Magnusson, Swedish ''Dota 2'' player * S4 (military), a logistics officer within military units Places * County Route S4 (California), a road in San Diego, California Science and mathematics Mathematics * S4 algebra, a variety of modal algebras, also called Interior algebra * Tetrahedral symmetry, the symmetric group S4 * S4 (modal logic), a normal modal logic Chemistry * S4: Keep away from living quarters, a safety phrase in chemistry * Tetrasulfur (S4), an allotrope of sulfur * Andarine (S-4), a selective androgen receptor modulator and experimental drug Biology * Fourth heart sound, or S4, an abnormal heart sound often indicative of congestive heart failure or cor pulmonale * Fourth sacrum of the vertebral column in human anatomy * Sacral spinal nerve 4, a spinal nerve of the sacral segment Technology * S (programming language) version 4 * Hibernation a sleeping state in a computer * SG2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. They are lustrous metals with good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Electrons
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell. An atom with a closed shell of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the relativel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Augmentation (geometry)
In geometry, a Johnson solid, sometimes also known as a Johnson–Zalgaller solid, is a convex polyhedron whose faces are regular polygons. They are sometimes defined to exclude the uniform polyhedrons. There are ninety-two solids with such a property: the first solids are the pyramids, cupolas, and a rotunda; some of the solids may be constructed by attaching with those previous solids, whereas others may not. Definition and background A Johnson solid is a convex polyhedron whose faces are all regular polygons. The convex polyhedron means as bounded intersections of finitely many half-spaces, or as the convex hull of finitely many points. Although there is no restriction that any given regular polygon cannot be a face of a Johnson solid, some authors required that Johnson solids are not uniform. This means that a Johnson solid is not a Platonic solid, Archimedean solid, prism, or antiprism. A convex polyhedron in which all faces are nearly regular, but some are not pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Icosahedron
In geometry, an icosahedron ( or ) is a polyhedron with 20 faces. The name comes . The plural can be either "icosahedra" () or "icosahedrons". There are infinitely many non- similar shapes of icosahedra, some of them being more symmetrical than others. The best known is the ( convex, non- stellated) regular icosahedron—one of the Platonic solids—whose faces are 20 equilateral triangles. Regular icosahedra There are two objects, one convex and one nonconvex, that can both be called regular icosahedra. Each has 30 edges and 20 equilateral triangle faces with five meeting at each of its twelve vertices. Both have icosahedral symmetry. The term "regular icosahedron" generally refers to the convex variety, while the nonconvex form is called a ''great icosahedron''. Convex regular icosahedron The convex regular icosahedron is usually referred to simply as the ''regular icosahedron'', one of the five regular Platonic solids, and is represented by its Schläfli symbol , contai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edge-contracted Icosahedron
In geometry, an edge-contracted icosahedron is a polyhedron with octadecahedron, 18 triangular face (geometry), faces, 27 Edge (geometry), edges, and 11 Vertex (geometry), vertices. Construction It can be constructed from the regular icosahedron, with one edge contraction, removing one vertex, 3 edges, and 2 faces. This contraction distorts the circumscribed sphere original vertices. With all equilateral triangle faces, it has 2 sets of 3 coplanar equilateral triangles (each forming a half-hexagon), and thus is not a Johnson solid. If the sets of three coplanar triangles are considered a single face (called a triamond), it has 10 vertices, 22 edges, and 14 faces, 12 triangles and 2 triamonds. It may also be described as having a hybrid square (geometry), square-pentagonal antiprismatic core (an antiprismatic core with one square base and one pentagonal base); each base is then augmentation (geometry), augmented with a pyramid (geometry), pyramid. Related polytopes The diss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gyroelongated Square Bipyramid
In geometry, the gyroelongated square bipyramid is a polyhedron with 16 triangular faces. it can be constructed from a square antiprism by attaching two equilateral square pyramid, equilateral square pyramids to each of its square faces. The same shape is also called hexakaidecadeltahedron, heccaidecadeltahedron, or tetrakis square antiprism; these last names mean a polyhedron with 16 triangular faces. It is an example of deltahedron, and of a Johnson solid. The dual polyhedron of the gyroelongated square bipyramid is a square truncated trapezohedron with eight pentagons and two squares as its faces. The gyroelongated square pyramid appears in chemistry as the basis for the bicapped square antiprismatic molecular geometry, and in mathematical optimization as a solution to the Thomson problem. Construction Like other Gyroelongated bipyramid, gyroelongated bipyramids, the gyroelongated square bipyramid can be constructed by attaching two Equilateral square pyramid, equilateral sq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triaugmented Triangular Prism
The triaugmented triangular prism, in geometry, is a convex polyhedron with 14 equilateral triangles as its faces. It can be constructed from a triangular prism by attaching equilateral square pyramids to each of its three square faces. The same shape is also called the tetrakis triangular prism, tricapped trigonal prism, tetracaidecadeltahedron, or tetrakaidecadeltahedron; these last names mean a polyhedron with 14 triangular faces. It is an example of a deltahedron, composite polyhedron, and Johnson solid. The edges and vertices of the triaugmented triangular prism form a maximal planar graph with 9 vertices and 21 edges, called the Fritsch graph. It was used by Rudolf and Gerda Fritsch to show that Alfred Kempe's attempted proof of the four color theorem was incorrect. The Fritsch graph is one of only six graphs in which every Neighbourhood (graph theory), neighborhood is a 4- or 5-vertex cycle. The dual polyhedron of the triaugmented triangular prism is an associahedron, a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |