|
Omoconazole
Omoconazole is an azole antifungal drug. Omonocazole is not available in the United States and Canada. In other countries, it is used to treat cutaneous candidiasis, dermatophytosis, pityriasis versicolor Pityriasis commonly refers to flaking (or scaling) of the skin. The word comes from the Greek πίτυρον 'bran'. Classification Types include: * Pityriasis alba, dry, fine-scaled, pale patches on the face * Pityriasis lichenoides chronica, c .... References Chlorobenzene derivatives Phenol ethers Imidazole antifungals Lanosterol 14α-demethylase inhibitors 4-Chlorophenyl compounds {{antimicrobial-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifungal Drug
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC). The evolution of antifungal resistance is a growing threat to health globally. Routes of administration Ocular Indicated when the fungal infection is located in the eye. There is currently only one ocular antifungal available: natamycin. However, various other antifungal agents could be compounded in this formulation. Intrathecal Used occasionally when there's an infection of the central nervous system and other systemic options cannot reach the concentration required in that region for therapeutic benefit. Example(s): amphotericin B. Vaginal This may be used to treat some fungal inf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azole
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring. Their names originate from the Hantzsch–Widman nomenclature. The parent compounds are aromatic and have two double bonds; there are successively redox, reduced analogs (azolines and azolidines) with fewer. One, and only one, lone pair of electrons from each heteroatom in the ring is part of the aromatic bonding in an azole. Names of azoles maintain the prefix upon reduction (e.g., pyrazoline, pyrazolidine). The numbering of ring atoms in azoles starts with the heteroatom that is not part of a double bond, and then proceeds towards the other heteroatom. Imidazole and other five-membered aromatic heterocyclic systems with two nitrogens are extremely common in nature and form the core of many biomolecules, such as histidine. Compound classes ;Nitrogen only Imidazol.svg, Imidazole Pyrazol.svg, Pyrazol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
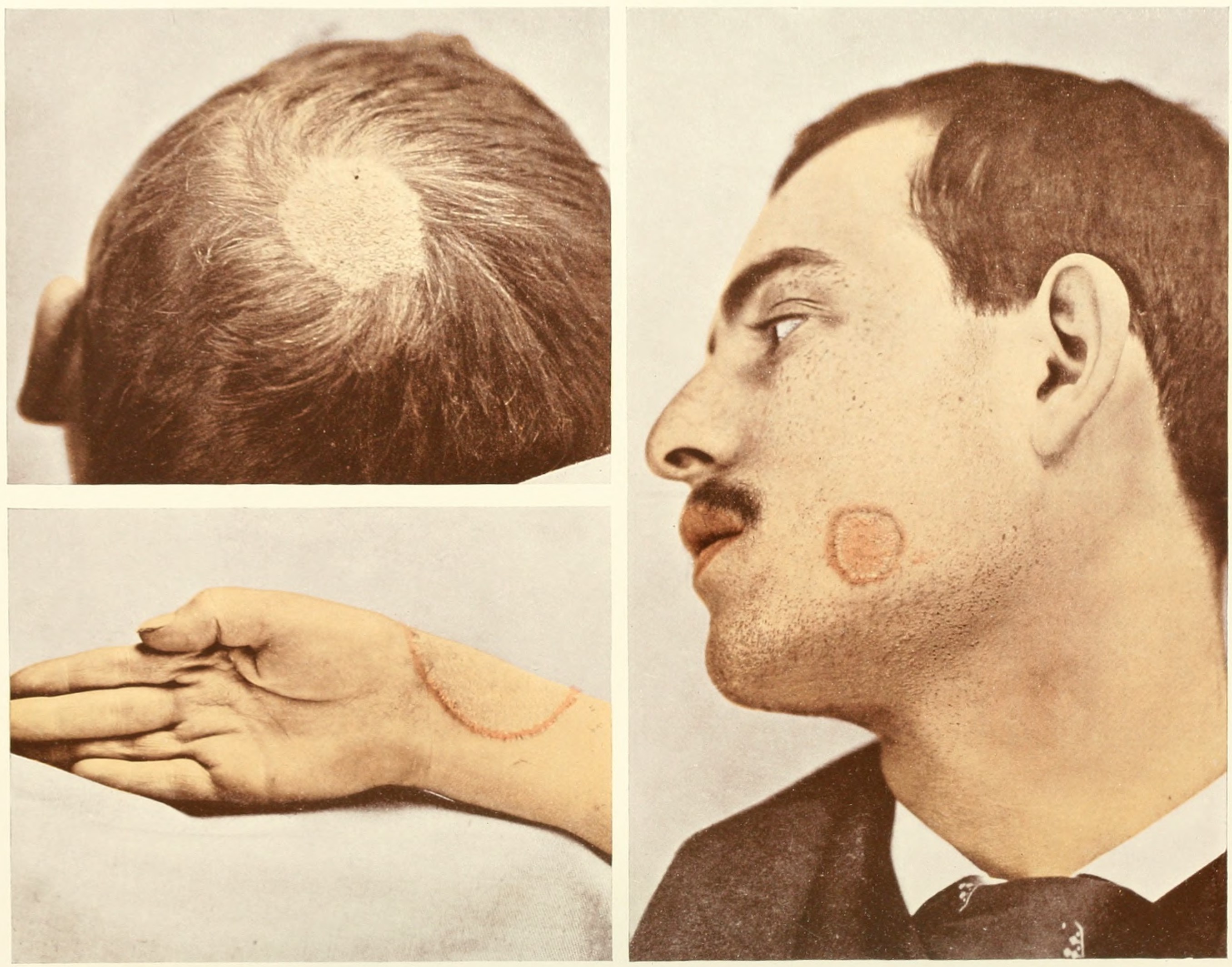
Cutaneous Candidiasis
Candidiasis is a fungal infection due to any species of the genus ''Candida'' (a yeast). When it affects the mouth, in some countries it is commonly called thrush. Signs and symptoms include white patches on the tongue or other areas of the mouth and throat. Other symptoms may include soreness and problems swallowing. When it affects the vagina, it may be referred to as a yeast infection or thrush. Signs and symptoms include genital itching, burning, and sometimes a white "cottage cheese-like" discharge from the vagina. Yeast infections of the penis are less common and typically present with an itchy rash. Very rarely, yeast infections may become invasive, spreading to other parts of the body. This may result in fevers, among other symptoms. Finally, candiasis of the esophagus is an important risk factor for contracting esophageal cancer in individuals with achalasia. More than 20 types of ''Candida'' may cause infection with ''Candida albicans'' being the most common. Infec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dermatophytosis
Dermatophytosis, also known as tinea and ringworm, is a fungal infection of the skin (a dermatomycosis), that may affect skin, hair, and nails. Typically it results in a red, itchy, scaly, circular rash. Hair loss may occur in the area affected. Symptoms begin four to fourteen days after exposure. The types of dermatophytosis are typically named for area of the body that they affect. Multiple areas can be affected at a given time. About 40 types of fungus can cause dermatophytosis. They are typically of the '' Trichophyton'', '' Microsporum'', or '' Epidermophyton'' type. Risk factors include using public showers, contact sports such as wrestling, excessive sweating, contact with animals, obesity, and poor immune function. Ringworm can spread from other animals or between people. Diagnosis is often based on the appearance and symptoms. It may be confirmed by either culturing or looking at a skin scraping under a microscope. Prevention is by keeping the skin dry, not w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pityriasis Versicolor
Pityriasis commonly refers to flaking (or scaling) of the skin. The word comes from the Greek πίτυρον 'bran'. Classification Types include: * Pityriasis alba, dry, fine-scaled, pale patches on the face * Pityriasis lichenoides chronica, caused by a hypersensitivity reaction to infectious agents * Pityriasis lichenoides et varioliformis acuta, a disease of the immune system * Pityriasis rosea, a type of skin rash ** Pityriasis circinata, * Pityriasis rubra pilaris, reddish-orange patches (Latin: ''rubra'') on the skin * Pityriasis versicolor, a skin eruption on the trunk and proximal extremities, usually caused by a fungus * Dandruff, historically called ''Pityriasis capitis'' * Pityriasis amiantacea, condition of the scalp in which thick tenaciously adherent scale infiltrates and surrounds the base of a group of scalp hairs See also * Desquamation * List of cutaneous conditions Many skin conditions affect the human integumentary system—the organ system covering t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorobenzene Derivatives
Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. Uses The major use of chlorobenzene is as a precursor for further intermediates such as nitrophenols, nitroanisole, chloroaniline, and phenylenediamines, which are used in the production of herbicides, dyestuffs, chemicals for rubber, and pharmaceuticals. It is also used as a high-boiling solvent in industrial and laboratory applications, for materials such as oils, waxes, resins, and rubber. Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated and used as intermediates in production of other chemicals. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Ethers
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole Antifungals
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ring, that exists in two equivalent tautomeric forms because hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanosterol 14α-demethylase Inhibitors
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, having a role in maintaining health of the lens. Lanosterol is the precursor to cholesterol. Biosynthesis The biosynthesis of lanosterol has been intensively investigated. Elaboration of lanosterol under enzyme catalysis leads to other steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol. Research as an eye drop supplement As a molecule naturally enriched in the eye lens, lanosterol is a component involved in maintenance of lens clarity. Its proposed mechanism of action is to inhibit the aggregation of crystallin proteins, which contribute to the clouding of vision by forming cataracts. Lanosterol is under research for its potential as a therapeutic additive in eye drops to inhibit the aggregation of crystallin p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |