|
Non-classical Ion
Nonclassical carbocations are stabilized by charge delocalization from contributions of neighbouring or bonds, which can form bridged intermediates or transition states. Nonclassical ions have been extensively studied with the 2-norbornyl system, which as “naked” ion unambiguously exhibit such a bridged structure. The landmark of nonclassical ions are unexpectedly fast solvolysis rates and large differences between epimeric esters. Such behaviour is not restricted to 2-norbornyl esters, as has been shown with some cyclopentyl and steroidal esters with the tosyloxy leaving group. Substitution reactions of secondary esters occur by SN2- or SN1-like mechanisms. Only in highly polar solvents such as hexafluoroisopropanol (HFIP) of low nucleophilicity one can expect a nearly same uniform SN1-like mechanism. The solvolysis of several cyclopentyl and steroidal esters show that large solvolysis rates and differences between epimers can occur which surpass those of the 2-norborn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocations
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
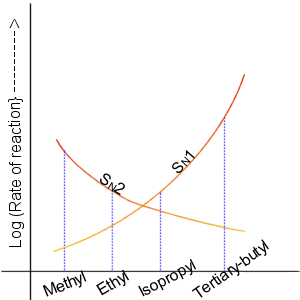
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocations
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration. Solubility of solid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neighbouring Group Participation
In organic chemistry, neighbouring group participation (NGP, also known as anchimeric assistance) has been defined by the International Union of Pure and Applied Chemistry (IUPAC) as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a pi bond contained within the parent molecule but not conjugated with the reaction centre. When NGP is in operation it is normal for the reaction rate to be increased. It is also possible for the stereochemistry of the reaction to be abnormal (or unexpected) when compared with a ''normal'' reaction. While it is possible for neighbouring groups to influence many reactions in organic chemistry (''e.g.'' the reaction of a diene such as 1,3-cyclohexadiene with maleic anhydride normally gives the endo isomer because of a secondary effect ) this page is limited to neighbouring group effects seen with carbocations and SN2 reactions. NGP by heteroatom lone pairs In this type of substitution reaction, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetics Via Nonclass Cations B
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to: Science and medicine * Kinetics (physics), the study of motion and its causes ** Rigid body kinetics, the study of the motion of rigid bodies * Chemical kinetics, the study of chemical reaction rates **Enzyme kinetics, the study of biochemical reaction rates catalysed by an enzyme *** Michaelis–Menten kinetics, the widely accepted general model of enzyme kinetics *** Goldbeter–Koshland kinetics, describe a steady-state solution for a 2-state biological system *** Langmuir–Hinshelwood kinetics **Receptor–ligand kinetics In biochemistry, receptor–ligand kinetics is a branch of chemical kinetics in which the kinetic species are defined by different non-covalent bindings and/or conformations of the molecules involved, which are denoted as '' receptor(s)'' and '' li ..., a branch of chemical kinetics in which the kinetic species are defined by different non-covalent bindings and/or c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvation
Solvation (or dissolution) describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration. Solubility of solid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-periplanar
In organic chemistry, anti-periplanar, or antiperiplanar, describes the bond angle in a molecule. In this conformer, the dihedral angle of the bond and the bond is greater than +150° or less than −150° (Figures 1 and 2). Anti-periplanar is often used in textbooks to mean strictly anti-coplanar, with an dihedral angle of 180° (Figure 3). In a Newman projection, the molecule will be in a staggered arrangement with the anti-periplanar functional groups pointing up and down, 180° away from each other (see Figure 4). Figure 5 shows 2-chloro-2,3-dimethylbutane in a sawhorse projection with chlorine and a hydrogen anti-periplanar to each other. Syn-periplanar or synperiplanar is similar to anti-periplanar. In the syn-periplanar conformer, the A and D are on the same side of the plane of the bond, with the dihedral angle of and between +30° and −30° (see Figure 2). Molecular orbitals An important factor in the antiperiplanar conformer is the interaction between molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |