|
Nitrides
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common. Synthesis of inorganic metal nitrides is challenging because nitrogen gas (N2) is not very reactive at low temperatures, but it becomes more reactive at higher temperatures. Therefore, a balance must be achieved between the low reactivity of nitrogen gas at low temperatures and the entropy driven formation of N2 at high temperatures. However, synthetic methods for nitrides are growing more sophisticated and the materials are of increasing technological rele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Nitride
Titanium nitride (TiN; sometimes known as Tinite) is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties. Applied as a thin coating, TiN is used to harden and protect cutting and sliding surfaces, for decorative purposes (due to its golden appearance), and as a non-toxic exterior for medical implants. In most applications a coating of less than is applied. Characteristics TiN has a Vickers hardness of 1800–2100, a modulus of elasticity of 251 GPa, a thermal expansion coefficient of 9.35 K−1, and a superconducting transition temperature of 5.6 K. TiN will oxidize at 800 °C in a normal atmosphere. TiN has a brown color, and appears gold when applied as a coating. It is chemically stable at 20 °C, according to laboratory tests, but can be slowly attacked by concentrated acid solutions with rising temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium Nitride
Beryllium nitride, Be3N2, is a nitride of beryllium. It can be prepared from the elements at high temperature (1100–1500 °C);Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier unlike beryllium azide or BeN6, it decomposes in vacuum into beryllium and nitrogen. It is readily hydrolysed forming beryllium hydroxide and ammonia. It has two polymorphic forms cubic α-Be3N2 with a defect anti-fluorite structure, and hexagonal β-Be3N2. It reacts with silicon nitride, Si3N4 in a stream of ammonia at 1800–1900 °C to form BeSiN2. Preparation Beryllium nitride is prepared by heating beryllium metal powder with dry nitrogen in an oxygen-free atmosphere in temperatures between 700 and 1400 °C. : 3Be + N2 → Be3N2 Uses It is used in refractory ceramicsHugh O. Pierson, 1996, Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing, and Applications, William Andrew Inc., as well as in nuclear reactors and to pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Nitride
Calcium nitride is the inorganic compound with the chemical formula Ca3 N2. It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered. Structure α-Calcium nitride adopts an anti-bixbyite structure, similar to Mn2O3, except that the positions of the ions are reversed: calcium (Ca2+) take the oxide (O2−) positions and nitride ions (N3−) the manganese (Mn3+). In this structure, Ca2+ occupies tetrahedral sites, and the nitride centres occupy two different types of octahedral sites. Synthesis and reactions Calcium nitride is formed along with the oxide, CaO, when calcium burns in air. It can be produced by direct reaction of the elements: :3 Ca + N2 → Ca3N2 It reacts with water or even the moisture in air to give ammonia and calcium hydroxide: :Ca3N2 + 6 H2O → 3 Ca(OH)2 + 2 NH3 Like sodium oxide Sodium oxide is a chemical compound with the formula Na2 O. It is used in ceramics and glasses. It is a white solid but the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
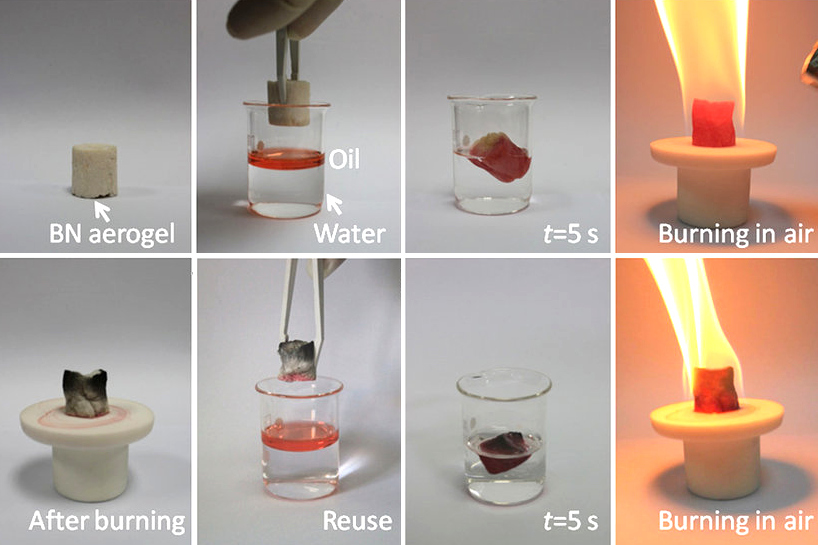
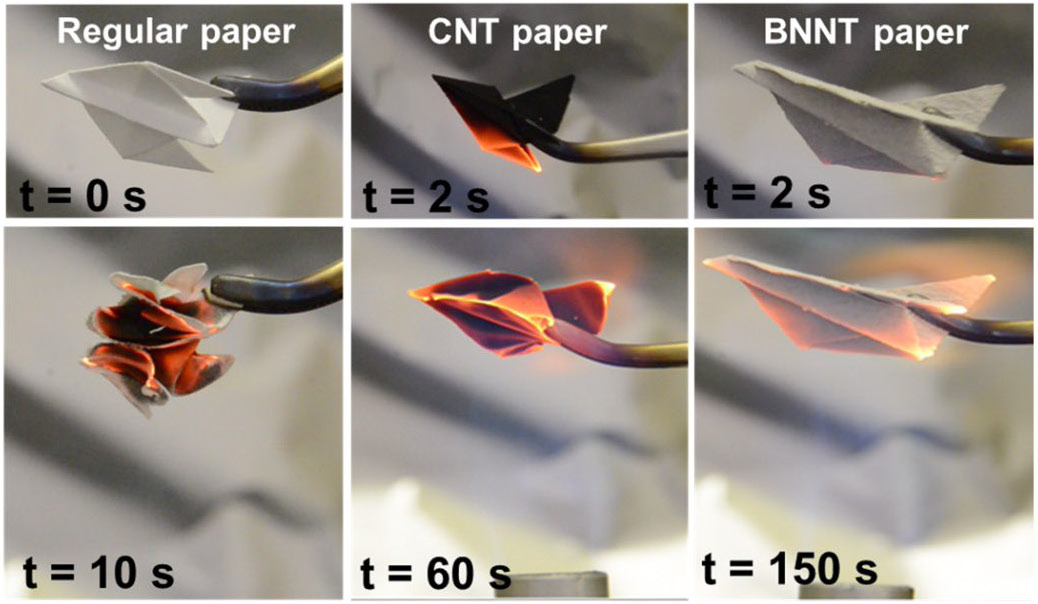
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitride
Sodium nitride is the inorganic compound with the chemical formula Na3N. In contrast to lithium nitride and some other nitrides, sodium nitride is an extremely unstable alkali metal nitride. It can be generated by combining atomic beams of sodium and nitrogen deposited onto a low-temperature sapphire substrate. 'Elusive Binary Compound Prepared'''Chemical & Engineering News'' 80 No. 20 (20 May 2002) It readily decomposes into its elements: :2 Na3N -> 6 Na + N2 Synthesis Sodium nitride can be synthesized in two different ways: by the thermal decomposition Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is re ... of NaNH2 or by the direct reaction of the elements. The most common way to successfully synthesize sodium nitride has been done by Dieter Fischer & Martin Jansen and Grigori Va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Nitride
Potassium nitride is an unstable chemical compound. Several syntheses were erroneously claimed in the 19th century, and by 1894 it was assumed that it did not exist. However, a synthesis of this compound was claimed in 2004. It is observed to have the anti- structure below , although a -type structure should be more stable. Above this temperature, it converts to an orthorhombic phase. This compound was produced by the reaction of potassium metal and liquid nitrogen at under vacuum: :6K + N2 → 2K3N This compound decomposes back into potassium and nitrogen at room temperature. This compound is unstable due to steric hindrance Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions .... References Potassium compounds Nitrides {{Chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Nitride
Strontium nitride, Sr3N2, is produced by burning strontium metal in air (resulting in a mixture with strontium oxide) or in nitrogen. Like other metal nitrides, it reacts with water to give strontium hydroxide and ammonia: :Sr3N2 + 6 H2O → 3 Sr(OH)2 + 2 NH3 See also * Beryllium nitride * Magnesium nitride * Calcium nitride Calcium nitride is the inorganic compound with the chemical formula Ca3 N2. It exists in various forms (isomorphs), α-calcium nitride being more commonly encountered. Structure α-Calcium nitride adopts an anti-bixbyite structure, similar to ... * Barium nitride References Nitrides Strontium compounds {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Nitride
Magnesium nitride, which possesses the chemical formula Mg3N2, is an inorganic compound of magnesium and nitrogen. At room temperature and pressure it is a greenish yellow powder. Preparation * By passing dry nitrogen over heated magnesium: ::\begin\\ \ce\\ \end * or ammonia: ::\begin\\ \ce\\ \end History When isolating argon, William Ramsay passed dry air over copper to remove oxygen and over magnesium to remove the nitrogen, forming magnesium nitride. Chemistry Magnesium nitride reacts with water to produce magnesium hydroxide and ammonia gas, as do many metal nitrides. :Mg3N2(s) + 6 H2O(l) → 3 Mg(OH)2(aq) + 2 NH3(g) In fact, when magnesium is burned in air, some magnesium nitride is formed in addition to the principal product, magnesium oxide. Thermal decomposition of magnesium nitride gives magnesium and nitrogen gas (at 700-1500 °C). At high pressures, the stability and formation of new nitrogen-rich nitrides (N/Mg ratio equal or greater to one) were sugge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Nitride
Gallium nitride () is a binary III/ V direct bandgap semiconductor commonly used in blue light-emitting diodes since the 1990s. The compound is a very hard material that has a Wurtzite crystal structure. Its wide band gap of 3.4 eV affords it special properties for applications in optoelectronic, high-power and high-frequency devices. For example, GaN is the substrate which makes violet (405 nm) laser diodes possible, without requiring nonlinear optical frequency-doubling. Its sensitivity to ionizing radiation is low (like other group III nitrides), making it a suitable material for solar cell arrays for satellites. Military and space applications could also benefit as devices have shown stability in high radiation environments. Because GaN transistors can operate at much higher temperatures and work at much higher voltages than gallium arsenide (GaAs) transistors, they make ideal power amplifiers at microwave frequencies. In addition, GaN offers promising charac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
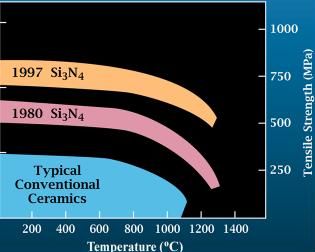
Silicon Nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot . It is very hard (8.5 on the mohs scale). It has a high thermal stability with strong optical nonlinearities for all-optical applications. Production Silicon nitride is prepared by heating powdered silicon between 1300 °C and 1400 °C in a nitrogen atmosphere: :3 Si + 2 → The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. Without an iron catalyst, the reaction is complete after several hours (~7), when no further weight increase due to nitrogen absorption (per gram of silicon) is detected. In addition to , several other silicon nitride phases (with chemica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Nitride
Lithium nitride is a compound with the formula Li3N. It is the only stable alkali metal nitride. The solid has a reddish-pink color and high melting point. Preparation and handling Lithium nitride is prepared by direct combination of elemental lithium with nitrogen gas: :6 Li + N2 → 2 Li3N Instead of burning lithium metal in an atmosphere of nitrogen, a solution of lithium in liquid sodium metal can be treated with N2. Lithium nitride reacts violently with water to produce ammonia: :Li3N + 3 H2O → 3 LiOH + NH3 Structure and properties ''alpha''-Li3N (stable at room temperature and pressure) has an unusual crystal structure that consists of two types of layers, one sheet has the composition Li2N− contains 6-coordinate N centers and the other sheet consists only of lithium cations. Two other forms are known: ''beta''-Lithium nitride, formed from the alpha phase at has the sodium arsenide (Na3As) structure; ''gamma''-Lithium nitride (same structure as Li3Bi) forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |