|
Nitrogen-13
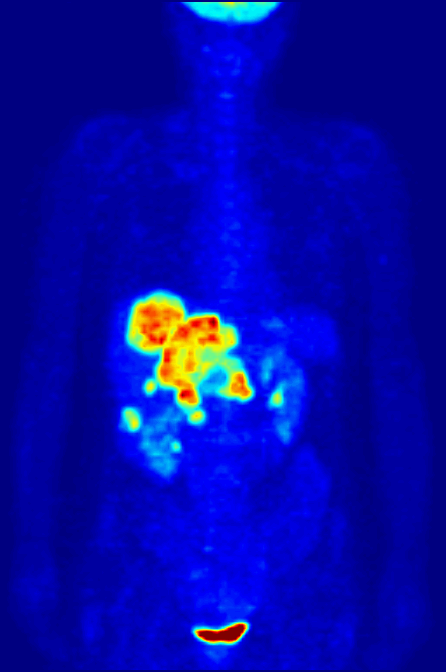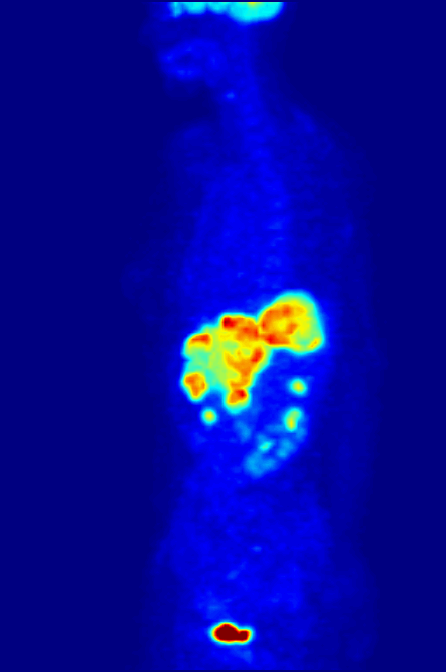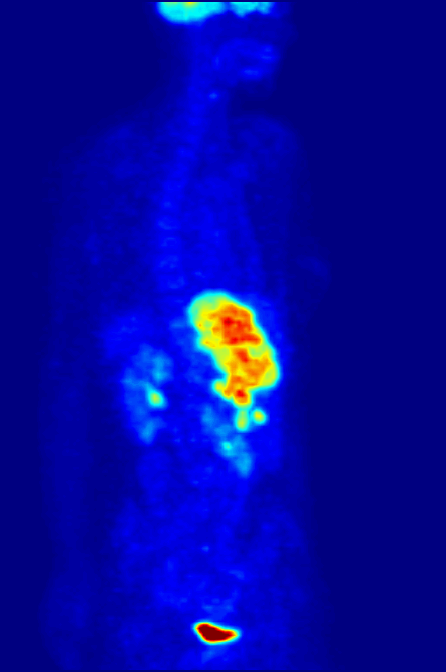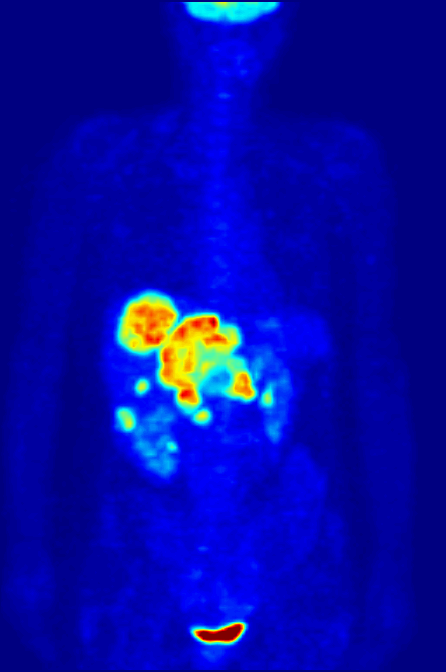
Nitrogen-13 (13N) is a radioisotope of nitrogen used in positron emission tomography (PET). It has a half-life of a little under ten minutes, so it must be made at the PET site. A cyclotron may be used for this purpose. Nitrogen-13 is used to tag ammonia molecules for PET myocardial perfusion imaging. Production Nitrogen-13 is used in medical PET imaging in the form of 13N-labelled ammonia. It can be produced with a medical cyclotron, using a target of pure water with a trace amount of ethanol. The reactants are oxygen-16 (present as H2O) and a proton, and the products are nitrogen-13 and an alpha particle (helium-4). :1H + 16O → 13N + 4He The proton must be accelerated to have total energy greater than 5.66 MeV. This is the threshold energy for this reaction, as it is endothermic (i.e., the mass of the products is greater than the reactants, so energy needs to be supplied which is converted to mass). For this reason, the proton needs to carry extra energy to induce the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen-13
There are three known stable isotopes of oxygen (8O): , , and . Radioactive isotopes ranging from to have also been characterized, all short-lived. The longest-lived radioisotope is with a half-life of , while the shortest-lived isotope is the unbound with a half-life of , though half-lives have not been measured for the unbound heavy isotopes and . List of isotopes , -id=Oxygen-11 , , style="text-align:right" , 8 , style="text-align:right" , 3 , , [] , proton emission, 2p , , (3/2−) , , , -id=Oxygen-12 , , style="text-align:right" , 8 , style="text-align:right" , 4 , , , 2p , , 0+ , , , - , rowspan=3, , rowspan=3 style="text-align:right" , 8 , rowspan=3 style="text-align:right" , 5 , rowspan=3, , rowspan=3, , β+ () , , rowspan=3, (3/2−) , rowspan=3, , rowspan=3, , - , β+p () , , - , β+p,α ( , - , Can be used in NMR studies of metabolic pathways. , style="text-align:right" , 8 , style="text-align:right" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Nitrogen
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Thirteen radioisotopes are also known, with atomic masses ranging from 9 to 23, along with three nuclear isomers. All of these radioisotopes are short-lived, the longest-lived being nitrogen-13 with a half-life of . All of the others have half-lives shorter than ten seconds, with most of these being below 500 milliseconds. Most of the isotopes with atomic mass numbers below 14 decay to isotopes of carbon, while most of the isotopes with masses above 15 decay to isotopes of oxygen. The shortest-lived known isotope is nitrogen-10, with a half-life of , though the half-life of nitrogen-9 has not been measured exactly. List of isotopes , -id=Nitrogen-9 , , style="text-align:right" , 7 , style="text-align:right" , 2 , , , , -id=Nitrogen-14m , style="text-indent:1em" , , colspan="3" style="text-ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myocardial Perfusion Imaging
Myocardial perfusion imaging or scanning (also referred to as MPI or MPS) is a nuclear medicine procedure that illustrates the function of the heart muscle (myocardium). It evaluates many heart conditions, such as coronary artery disease (CAD), hypertrophic cardiomyopathy and heart wall motion abnormalities. It can also detect regions of myocardial infarction by showing areas of decreased resting perfusion. The function of the myocardium is also evaluated by calculating the left ventricular ejection fraction (LVEF) of the heart. This scan is done in conjunction with a cardiac stress test. The diagnostic information is generated by provoking controlled regional ischemia in the heart with variable perfusion. Planar techniques, such as conventional scintigraphy, are rarely used. Rather, single-photon emission computed tomography (SPECT) is more common in the US. With multihead SPECT systems, imaging can often be completed in less than 10 minutes. With SPECT, inferior and posterior ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen-14
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Thirteen radioisotopes are also known, with atomic masses ranging from 9 to 23, along with three nuclear isomers. All of these radioisotopes are short-lived, the longest-lived being nitrogen-13 with a half-life of . All of the others have half-lives shorter than ten seconds, with most of these being below 500 milliseconds. Most of the isotopes with atomic mass numbers below 14 decay to isotopes of carbon, while most of the isotopes with masses above 15 decay to isotopes of oxygen. The shortest-lived known isotope is nitrogen-10, with a half-life of , though the half-life of nitrogen-9 has not been measured exactly. List of isotopes , -id=Nitrogen-9 , , style="text-align:right" , 7 , style="text-align:right" , 2 , , ) , , rowspan=4, 1/2−# , rowspan=4, , rowspan=4, , - , β−n () , , - , � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNO Cycle
In astrophysics, the carbon–nitrogen–oxygen (CNO) cycle, sometimes called Bethe–Weizsäcker cycle, after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker, is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, the other being the proton–proton chain reaction (p–p cycle), which is more efficient at the Sun's core temperature. The CNO cycle is hypothesized to be dominant in stars that are more than 1.3 times as massive as the Sun. Unlike the proton-proton reaction, which consumes all its constituents, the CNO cycle is a catalytic cycle. In the CNO cycle, four protons fuse, using carbon, nitrogen, and oxygen isotopes as catalysts, each of which is consumed at one step of the CNO cycle, but re-generated in a later step. The end product is one alpha particle (a stable helium nucleus), two positrons, and two electron neutrinos. There are various alternative paths and catalysts involved in the CNO cycles, but all thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Emission
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (). Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus. An example of positron emission (β+ decay) is shown with magnesium-23 decaying into sodium-23: : → + + Because positron emission decreases proton number relative to neutron number, positron decay happens typically in large "proton-rich" radionuclides. Positron decay results in nuclear transmutation, changing an atom of one chemical element into an atom of an element with an atomic number that is less by one unit. Positron emission occurs extremely rarely in nature on Earth. Known instances include cosmic ray interactions and the decay of certain isotopes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body, such as: * Fluorodeoxyglucose ( 18F">sup>18FDG or FDG) is commonly used to detect cancer; * 18Fodium fluoride">sup>18Fodium fluoride (Na18F) is widely used for detecting bone formation; * Oxygen-15 (15O) is sometimes used to measure blood flow. PET is a common imaging technique, a medical scintillography technique used in nuclear medicine. A radiopharmaceutical—a radioisotope attached to a drug—is injected into the body as a tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is emitted, and when the positron interacts with an ordinary electron, the tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNO Cycle
In astrophysics, the carbon–nitrogen–oxygen (CNO) cycle, sometimes called Bethe–Weizsäcker cycle, after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker, is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, the other being the proton–proton chain reaction (p–p cycle), which is more efficient at the Sun's core temperature. The CNO cycle is hypothesized to be dominant in stars that are more than 1.3 times as massive as the Sun. Unlike the proton-proton reaction, which consumes all its constituents, the CNO cycle is a catalytic cycle. In the CNO cycle, four protons fuse, using carbon, nitrogen, and oxygen isotopes as catalysts, each of which is consumed at one step of the CNO cycle, but re-generated in a later step. The end product is one alpha particle (a stable helium nucleus), two positrons, and two electron neutrinos. There are various alternative paths and catalysts involved in the CNO cycles, but all thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |