|
Neutron Howitzer
A neutron howitzer is a neutron source that emits neutrons in a single direction. It was discovered in the 1930s that alpha radiation that strikes the beryllium nucleus would release neutrons. The high speed of the alpha is sufficient to overcome the relatively low Coulomb barrier of the beryllium nucleus, the repulsive force due to the positive charge of the nucleus, which contains only four protons, allowing for fusion of the two particles, releasing energetic neutrons. In 1930 Walther Bothe and Herbert Becker in Germany found that alpha particles striking light elements such as beryllium, boron, or lithium would release a highly penetrating radiation, at first believed to be gamma radiation, although it was more penetrating than any gamma rays known. The next important contribution was reported in 1932 by Irène Joliot-Curie and Frédéric Joliot in Paris, who showed that if this unknown radiation fell on paraffin wax or any other hydrogen-containing compound it ejected prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear power. Neutron source variables include the energy of the neutrons emitted by the source, the rate of neutrons emitted by the source, the size of the source, the cost of owning and maintaining the source, and government regulations related to the source. Small devices Spontaneous fission Some isotopes undergo spontaneous fission (SF) with Neutron emission, emission of neutrons. The most common spontaneous fission source is the isotope californium-252. 252Cf and all other SF neutron sources are made by irradiating uranium or a Transuranium element, transuranic element in a nuclear reactor, where neutrons are absorbed in the starting material and its subsequent reaction products, transmuting the starting material into the SF isotope. 252Cf n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fritz Strassman
Friedrich Wilhelm Strassmann (; 22 February 1902 – 22 April 1980) was a German chemist who, with Otto Hahn in December 1938, identified the element barium as a product of the bombardment of uranium with neutrons. Their observation was the key piece of evidence necessary to identify the previously unknown phenomenon of nuclear fission, as was subsequently recognized and published by Lise Meitner and Robert Frisch. In their second publication on nuclear fission in February 1939, Strassmann and Hahn predicted the existence and liberation of additional neutrons during the fission process, opening up the possibility of a nuclear chain reaction. Early life Friedrich Wilhelm (Fritz) Strassmann was born in Boppard, Germany, to Richard Strassmann and Julie Strassmann (née Bernsmann). He was the youngest of nine children. Growing up in Düsseldorf, he developed an interest in chemistry at a young age and conducted chemistry experiments in his parents' home. His family was of modest m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and nuclear weapons. Hahn and Lise Meitner discovered isotopes of the radioactive elements isotopes of radium, radium, Isotopes of thorium, thorium, isotopes of protactinium, protactinium and isotopes of uranium, uranium. He also discovered the phenomena of atomic recoil and nuclear isomerism, and pioneered rubidium–strontium dating. In 1938, Hahn, Meitner and Fritz Strassmann Discovery of nuclear fission, discovered nuclear fission, for which Hahn alone was awarded the 1944 Nobel Prize in Chemistry. A graduate of the University of Marburg, which awarded him a doctorate in 1901, Hahn studied under Sir William Ramsay at University College London and at McGill University in Montreal under Ernest Rutherford, where he discovered several new radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Howitzer
The howitzer () is an artillery weapon that falls between a cannon (or field gun) and a mortar. It is capable of both low angle fire like a field gun and high angle fire like a mortar, given the distinction between low and high angle fire breaks at 45 degrees or 800 mils (NATO). With their long-range capabilities, howitzers can be used to great effect in a battery formation with other artillery pieces, such as long-barreled guns, mortars, and rocket artillery. Howitzers were valued for their ability to fire explosive shells and incendiary materials into fortifications. Unlike mortars, which had fixed firing angles, howitzers could be fired at various angles, providing greater flexibility in combat. Throughout the 18th and 19th centuries, howitzers evolved to become more mobile and versatile. The introduction of rifling in the mid-19th century led to significant changes in howitzer design and usage. By the early 20th century, howitzers were classified into different categor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
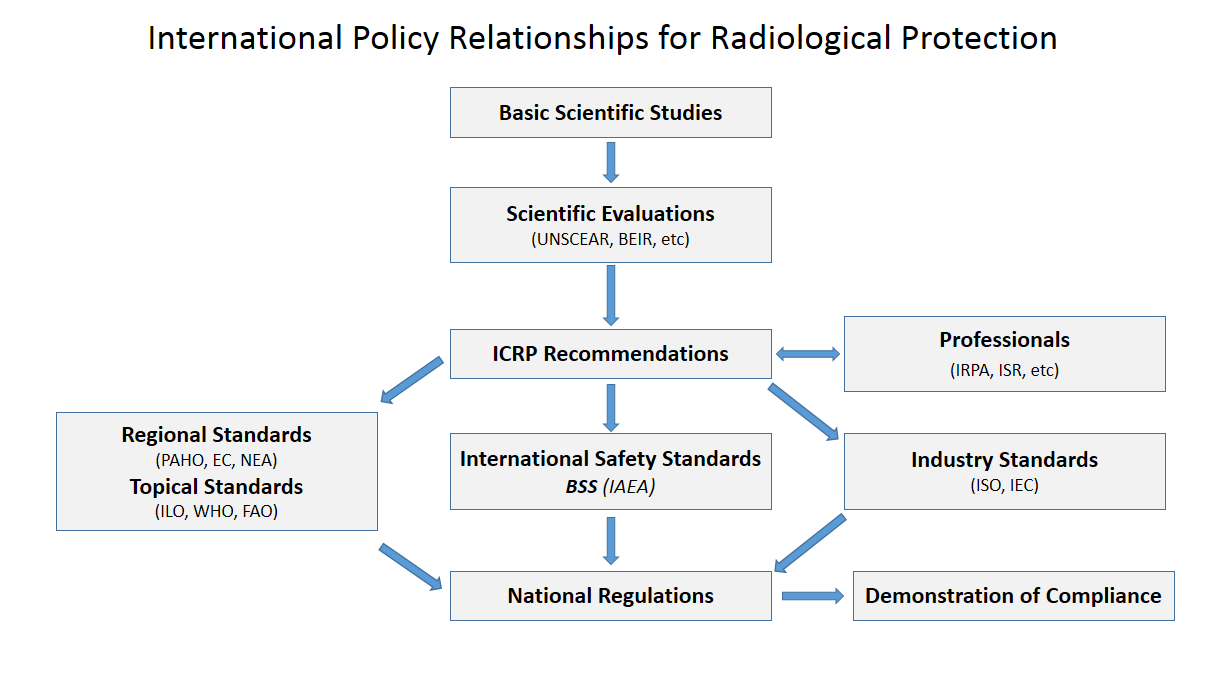
Radiation Shielding
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination. Ionizing radiation is widely used in industry and medicine, and can present a significant health hazard by causing microscopic damage to living tissue. There are two main categories of ionizing radiation health effects. At high exposures, it can cause "tissue" effects, also called "deterministic" effects due to the certainty of them happening, conventionally indicated by the unit gray and resulting in acute radiation syndrome. For low level exposures there can be statistically elevated risks of radiation-induced cancer, called "stochastic effects" due to the uncertainty of them happening, conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pu-239
Plutonium-239 ( or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years. Nuclear properties The nuclear properties of plutonium-239, as well as the ability to produce large amounts of nearly pure 239Pu more cheaply than highly enriched weapons-grade uranium-235, led to its use in nuclear weapons and nuclear power plants. The fissioning of an atom of uranium-235 in the reactor of a nuclear power plant produces two to three neutrons, and these neutrons can be absorbed by uranium-238 to produce plutonium-239 and other isotopes. Plutonium-239 can also absorb neutrons and fission along with the uranium-235 in a reactor. Of all the common nuclear fuels, 23 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Am-241
Americium-241 (Am, Am-241) is an isotope of americium. Like all isotopes of americium, it is radioactive, with a half-life of . Am is the most common isotope of americium as well as the most prevalent isotope of americium in nuclear waste. It is commonly found in ionization type smoke detectors and is a potential fuel for long-lifetime radioisotope thermoelectric generators (RTGs). Its common parent nuclides are β from Pu, EC from Cm, and α from Bk. Am is not fissile, but is fissionable, and the critical mass of a bare sphere is and a sphere diameter of . Americium-241 has a specific activity of . It is commonly found in the form of americium-241 dioxide (AmO). This isotope also has one meta state, Am, with an excitation energy of and a half-life of . The presence of Am in plutonium is determined by the original concentration of plutonium-241 and the sample age. Because of the low penetration of alpha radiation, americium-241 only poses a health risk when ingested o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ra-226
Radium-226 () is the longest-lived isotope of radium, with a half-life of 1600 years. It is an intermediate product in the decay chain of uranium-238; as such, it can be found naturally in uranium-containing minerals. Occurrence and decay occurs in the decay chain of uranium-238 (), which is the most common naturally occurring isotope of uranium. It undergoes alpha decay to radon-222, which is also radioactive; the decay chain ultimately terminates at lead-206. Because of its occurrence in the decay chain, exists naturally at low concentrations in uranium-containing minerals, soil, and groundwater. Historical uses Following its discovery by Marie Curie, Marie and Pierre Curie in 1898, radium (principally ) has had a number of uses. In the early 20th century, when the hazards of ionizing radiation, radiation were not well-known, radium was commonly used in consumer items such as toothpaste and hair creams. Radium was also formerly used as a radiation source for cancer treat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1,600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence. For this property, it was widely used in Self-luminous paint, self-luminous paints following its discovery. Of the Radionuclide, radioactive elements that occur in quantity, radium is considered particularly Toxicity, toxic, and it is Carcinogen, carcinogenic due to the radioactivity of both it and its immediate decay product radon as well as its tendency to B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Activity
Specific activity (symbol ''a'') is the activity per unit mass of a radionuclide and is a physical property of that radionuclide. It is usually given in units of becquerel per kilogram (Bq/kg), but another commonly used unit of specific activity is the curie per gram (Ci/g). In the context of radioactivity, activity or total activity (symbol ''A'') is a physical quantity defined as the number of radioactive transformations per second that occur in a particular radionuclide. The unit of activity is the ''becquerel'' (symbol Bq), which is defined equivalent to reciprocal seconds (symbol s−1). The older, non-SI unit of activity is the ''curie'' (Ci), which is radioactive decays per second. Another unit of activity is the ''rutherford'', which is defined as radioactive decays per second. The specific activity should not be confused with level of exposure to ionizing radiation and thus the exposure or absorbed dose, which is the quantity important in assessing the effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |