|
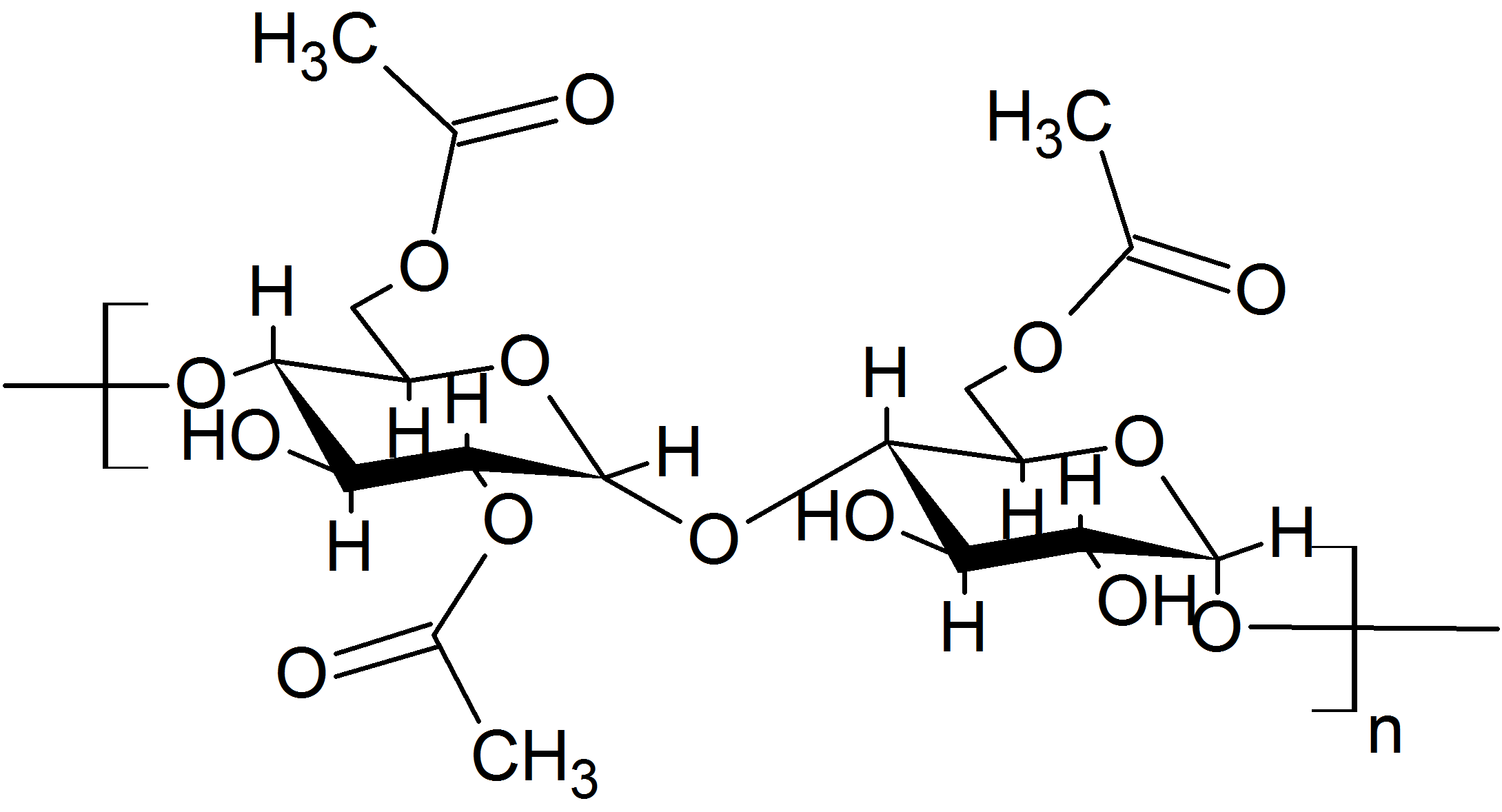
Modified Starch
Modified starch, also called starch derivatives, are prepared by physically, enzymatically, or chemically treating native starch to change its properties. Modified starches are used in practically all starch applications, such as in food products as a thickening agent, stabilizer or emulsifier; in pharmaceuticals as a disintegrant; or as binder in coated paper. They are also used in many other applications. Starches are modified to enhance their performance in different applications. Starches may be modified to increase their stability against excessive heat, acid, shear, time, cooling, or freezing; to change their texture; to decrease or increase their viscosity; to lengthen or shorten gelatinization time; or to increase their visco-stability. Modification methods Acid-treated starch (INS 1401), also called thin boiling starch, is prepared by treating starch or starch granules with inorganic acids, e.g. hydrochloric acid breaking down the starch molecule and thus redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Modified Food Starch
Modified starch, also called starch derivatives, are prepared by physically, enzymatically, or chemically treating native starch to change its properties. Modified starches are used in practically all starch applications, such as in food products as a thickening agent, stabilizer or emulsifier; in pharmaceuticals as a disintegrant; or as binder in coated paper. They are also used in many other applications. Starches are modified to enhance their performance in different applications. Starches may be modified to increase their stability against excessive heat, acid, shear, time, cooling, or freezing; to change their texture; to decrease or increase their viscosity; to lengthen or shorten gelatinization time; or to increase their visco-stability. Modification methods Acid-treated starch (INS 1401), also called thin boiling starch, is prepared by treating starch or starch granules with inorganic acids, e.g. hydrochloric acid breaking down the starch molecule and thus redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclodextrin
Cyclodextrins are a family of cyclic oligosaccharides, consisting of a macrocyclic ring of glucose subunits joined by α-1,4 glycosidic bonds. Cyclodextrins are produced from starch by enzymatic conversion. They are used in food, pharmaceutical, drug delivery, and chemical industries, as well as agriculture and environmental engineering. Cyclodextrins are composed of 5 or more α-D-glucopyranoside units linked 1->4, as in amylose (a fragment of starch). Typical cyclodextrins contain a number of glucose monomers ranging from six to eight units in a ring, creating a cone shape: * α (alpha)-cyclodextrin: 6 glucose subunits * β (beta)-cyclodextrin: 7 glucose subunits * γ (gamma)-cyclodextrin: 8 glucose subunits The largest well-characterized cyclodextrin contains 32 1,4-anhydroglucopyranoside units. Poorly-characterized mixtures, containing at least 150-membered cyclic oligosaccharides are also known. Applications Drug delivery Cyclodextrins are ingredients in more than 30 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltodextrin
Maltodextrin is a polysaccharide that is used as a food ingredient. It is produced from vegetable starch by partial hydrolysis and is usually found as a white hygroscopic spray-dried powder. Maltodextrin is easily digestible, being absorbed as rapidly as glucose and may be either moderately sweet or almost flavorless (depending on the degree of polymerisation). It can be found as an ingredient in a variety of processed foods. Structure Maltodextrin consists of D-glucose units connected in chains of variable length. The glucose units are primarily linked with α(1→4) glycosidic bonds, like that seen in the linear derivative of glycogen (after the removal of α1,6- branching). Maltodextrin is typically composed of a mixture of chains that vary from three to 17 glucose units long. Maltodextrins are classified by DE ( dextrose equivalent) and have a DE between 3 and 20. The higher the DE value, the shorter the glucose chains, the higher the sweetness, the higher the solubility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate ·5, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is the oldest and still most important chlorine-based bleach. Its corrosive properties, common availability, and reaction products make it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploit its caustic nature and its reactivity toward acids. An estimated 700,000 to 800,000 tonnes were produced in 2005. KOH is noteworthy as the precursor to most soft and liquid soaps, as well as numerous potassium-containing chemicals. It is a white solid that is dangerously corrosive. Properties and structure KOH exhibits high thermal stability. Because of this high stability and relatively low melting point, it is often melt-cast as pellets or rods, forms that have low surface area and convenient handling properties. These pellets become tacky in air because KOH is hygroscopic. Most commercial samples are ca. 90% pure, the remainder being water and carbonates. Its dissolution in water is strongly exothermic. Concentrated aqueous so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextrin
Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch and glycogen. Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds. Dextrins can be produced from starch using enzymes like amylases, as during digestion in the human body and during malting and mashing, or by applying dry heat under acidic conditions (pyrolysis or roasting). This procedure was first discovered in 1811 by Edme-Jean Baptiste Bouillon-Lagrange. The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavor, color and crispness. Dextrins produced by heat are also known as pyrodextrins. Starch hydrolyses during roasting under acidic conditions, and short-chained starch parts partially rebranch with α-(1,6) bonds to the degraded starch molecule. See also Maillard reaction. Dextrins are white, yellow, or brown powder that are partially or fully water-solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E-numbers
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today. Having a single unified list for food additives was first agreed upon in 1962 with food colouring. In 1964, the directives for preservatives were added, in 1970 antioxidants were added, in 1974 emulsifiers, stabilisers, thickeners and gelling agents were added as well. Numbering schemes The numbering scheme follows that of the International Numbering System (INS) as determined by the '' Codex Alimentarius'' committee, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water. Characteristics Commonly used mineral acids are sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3, they are also known as bench acids). Mineral acids range from superacids (perchloric acid) to very weak ones (boric acid). Mineral acids tend to be very soluble in water and insoluble in organic solvents. Mineral acids are used in many sectors of the chemical industry as feedstocks for the synthesis of other chemicals, both organic and inorganic. Large quantities of these acids – especially sulfuric acid, nitric acid, and hydrochloric acid – are manufactured for commercial use in large plants. Mineral acids are also used directly for their corrosive properties. For example, a dilute solution of hydrochloric acid is used f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |