|
Myristoylation
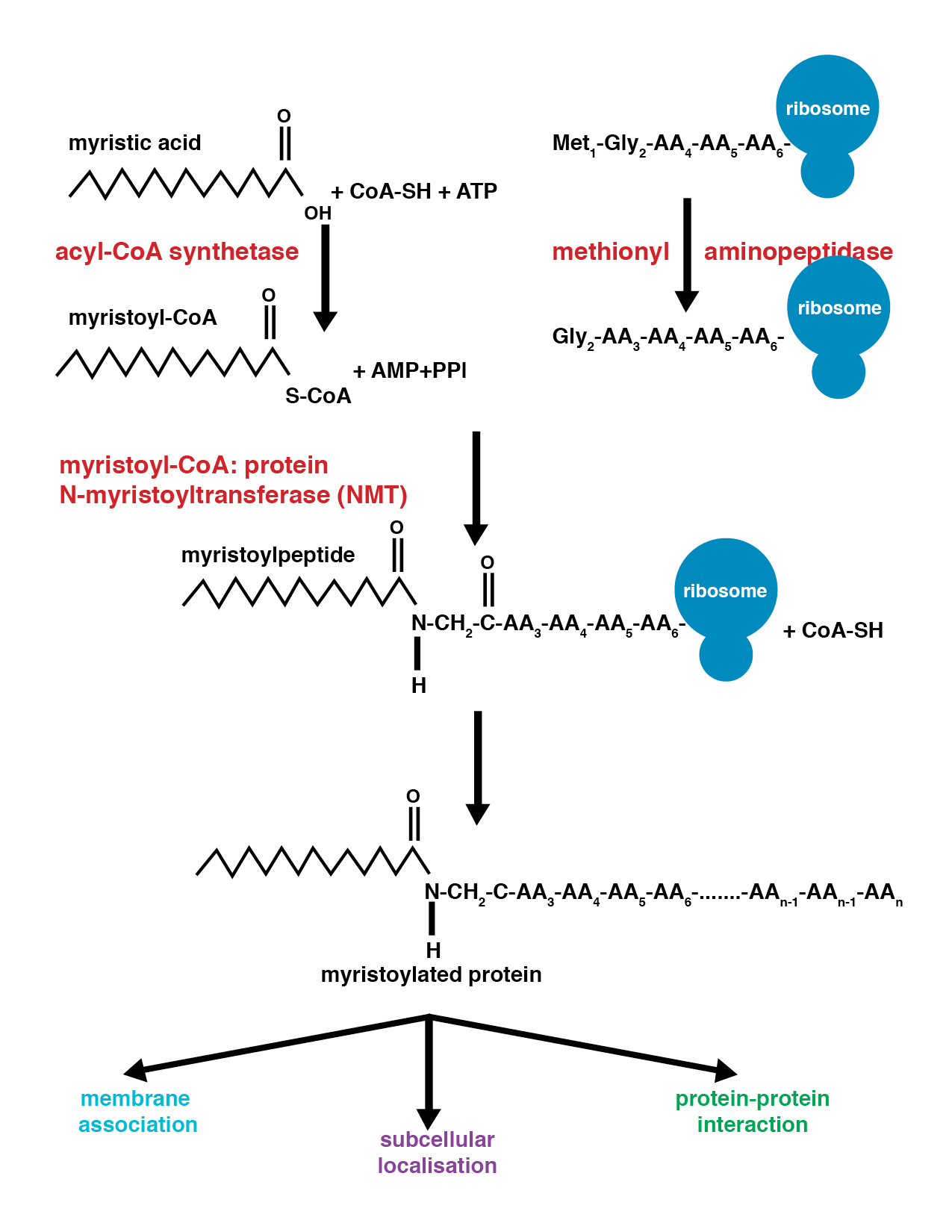
Myristoylation is a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached by an amide bond to the alpha-amino group of an ''N''-terminal glycine residue. Myristic acid is a 14-carbon saturated fatty acid (14:0) with the systematic name of ''n''-tetradecanoic acid. This modification can be added either co-translationally or post-translationally. ''N''-myristoyltransferase (NMT) catalyzes the myristic acid addition reaction in the cytoplasm of cells. This lipidation event is the most common type of fatty acylation and is present in many organisms, including animals, plants, fungi, protozoans and viruses. Myristoylation allows for weak protein–protein and protein–lipid interactions and plays an essential role in membrane targeting, protein–protein interactions and functions widely in a variety of signal transduction pathways. Discovery In 1982, Koiti Titani's lab identified an "''N''-terminal blocking group" on the catal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-myristoyltransferase 1
Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the ''NMT1'' gene. It belongs to the protein N-terminal methyltransferase and glycylpeptide N-tetradecanoyltransferase family of enzymes. This enzyme can be inhibited by N-myristoyltransferase inhibitors, NMT inhibitors. References Further reading * * * * * * * * * * * * * * * * * * * See also * Myristoylation * NMT2, N-myristoyltransferase 2 External links * EC 2.3.1 Human proteins {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biology), translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signal transduction, signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C-terminus, C- or N-terminus, N- termini. They can expand the chemical set of the 22 proteinogenic amino acid, amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMT2
Glycylpeptide N-tetradecanoyltransferase 2 known also as N-myristoyltransferase, is an enzyme (EC: 2.3.1.97) that in humans is encoded by the ''NMT2'' gene. Function N-myristoyltransferase (NMT) catalyzes the reaction of N-terminal myristoylation of many signaling proteins. It transfers myristic acid from myristoyl coenzyme A to the amino group of a protein's N-terminal glycine residue. Biochemical evidence indicates the presence of several distinct NMTs, varying in apparent molecular weight and /or subcellular distribution. The 496-amino acid of human NMT2 protein shares 77% and 96% sequence identity with human NMT1 and mouse Nmt2 comprise two distinct families of N-myristoyltransferases. Interactions NMT2 has been shown to interact with: * caspase 3 * MARCKS See also * N-myristoyltransferase 1 Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the ''NMT1'' gene. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycylpeptide N-tetradecanoyltransferase
In enzymology, a glycylpeptide N-tetradecanoyltransferase () is an enzyme that catalyzes the chemical reaction :tetradecanoyl-CoA + glycylpeptide \rightleftharpoons CoA + N-tetradecanoylglycylpeptide Thus, the two substrates of this enzyme are tetradecanoyl-CoA and glycylpeptide, whereas its two products are CoA and N-tetradecanoylglycylpeptide. It participates in the N-Myristoylation of proteins, and in vertebrates there are two isoenzymes NMT1 and NMT2. Besides tetradecanoyl-CoA, this enzyme is also capable of using modified versions of this substrate. In human retina, an even wider range of fatty acids, including 14:1 n–9, 14:2n–6, and 12:0, are accepted by the enzyme and grafted onto guanylate cyclase activators. This is mainly a result of a special set of fatty-acid-CoA substrates available in the retina. Nomenclature This enzyme belongs to the family of transferases, specifically those N-acyltransferases transferring groups other than aminoacyl groups (cd04301 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myristic Acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula . Its salts and esters are commonly referred to as myristates or tetradecanoates. The name of the acyl group derived from myristic acid is myristoyl or tetradecanoyl. The acid is named after the binomial name for nutmeg (''Myristica fragrans''), from which it was first isolated in 1841 by Lyon Playfair, 1st Baron Playfair, Lyon Playfair. Occurrence Nutmeg#Nutmeg butter, Nutmeg butter has 75% trimyristin, the triglyceride of myristic acid and a source from which it can be synthesised. Besides nutmeg, myristic acid is found in palm kernel oil, coconut oil, butterfat, 8–14% of bovine milk, and 8.6% of breast milk as well as being a minor component of many other animal fats. It is found in spermaceti, the crystallized fraction of oil from the sperm whale. It is also found in the rhizomes of the Iris (plant), Iris, including Orris root. Chemical behaviour Myristic acid acts a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase A
In cell biology, protein kinase A (PKA) is a family of serine-threonine kinases whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase (AMP-activated protein kinase). History Protein kinase A, more precisely known as adenosine 3',5'-monophosphate (cyclic AMP)-dependent protein kinase, abbreviated to PKA, was discovered by chemists Edmond H. Fischer and Edwin G. Krebs in 1968. They won the Nobel Prize in Physiology or Medicine in 1992 for their work on phosphorylation and dephosphorylation and how it relates to PKA activity. PKA is one of the most widely researched Protein kinase, protein kinases, in part because of its uniqueness; out of 540 different protein kinase genes that make up the human kinome, only one other protein kinase, casein kinase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...s combine to form a larger molecule called the '' adduct''.. An addition reaction is limited to chemical compounds that have multiple bonds. Examples include a molecule with a carbon–carbon double bond (an alkene) or a triple bond (an alkyne). Another example is a compound that has rings (which are also considered points of unsaturation). A molecule that has carbon— heteroatom double bonds, such as a carbonyl group () or imine group (), can undergo an addition reaction because its double-bond. An addition reaction is the reverse of an elimination reaction, in which one molecule divides into two or more molecules. For insta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix). The alpha helix is the most common structural arrangement in the Protein secondary structure, secondary structure of proteins. It is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. The alpha helix has a right-handed helix conformation in which every backbone amino, N−H group hydrogen bonds to the backbone carbonyl, C=O group of the amino acid that is four residue (biochemistry), residues earlier in the protein sequence. Other names The alpha helix is also commonly called a: * Pauling–Corey–Branson α-helix (from the names of three scientists who described its structure) * 3.613-helix because there are 3.6 amino acids in one ring, with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) Discovery ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone chain, backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of peptide, polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformational isomerism, conformation. The supramolecular association of β-sheets has been implicated in the formation of the Amyloid fibril, fibrils and Amyloid plaques, protein aggregates observed in amyloidosis, Alzheimer's disease and other Proteinopathy, proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid monomer may also be called a ''residue'', which indicates a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions, such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |