|
Murahashi Coupling
The Murahashi Coupling is a cross coupling reaction. The coupling partners are organolithiums and organic halides. Transition metal catalysts are required. The reaction was first reported by Shun-Ichi Murahashi in 1974. This reaction is notable for using organolithiums as opposed to other cross-coupling reactions which utilize various metal-carbon compounds (metal = tin, magnesium, boron, silicon, zinc). Since the production of these other coupling reagents relies heavily upon organolithiums (especially in the case of organozinc and organomagnesium compounds), in bypassing these intermediates, this process is much more efficient. It has further been shown that the Murahashi reaction proceeds with greater selectivity, faster reaction times, and lower reaction temperatures than other similar coupling reactions while maintaining similar or higher yields. History Murahashi first reported C(sp2)—C(sp3) coupling in 1974. These reactions were not metal catalyzed but were promot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross Coupling Reaction
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reaction type is this: : (R, R' = organic fragments, usually aryl; M = main group center such as Li or MgX; X = halide) These reactions are used to form carbon–carbon bonds but also carbon-heteroatom bonds. Cross-coupling reaction are a subset of coupling reactions. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism Many mechanisms exist reflecting the myriad types of cross-couplings, including those that do not require metal catalysts. Often, however, cross-coupling refers to a metal-catalyzed reaction of a nucleophilic partner with an electrophilic partner. In such cases, the mechanism generally involves reductive elimin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hiyama Coupling
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds (C-C bonds). This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Hatanaka as a method to form carbon-carbon bonds synthetically with chemo- and regioselectivity. The Hiyama coupling has been applied to the synthesis of various natural products. :\begin\\ \ce \end :* R: aryl, alkenyl or alkynyl :* R': aryl, alkenyl, alkynyl or alkyl :* R'': Cl, F or alkyl :* X: Cl, Br, I or OTf Reaction history The Hiyama coupling was developed to combat the issues associated with other organometallic reagents. The initial reactivity of organosilicon was not actually first reported by Hiyama, as Kumada reported a coupling reaction using organofluorosilicates shown below. Organosilanes were then discovered, by Hiyama, to have reactivity when activated by a fluoride source. This reactivity, when combined with a palladium s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kumada Coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki reaction, Suzuki, Sonogashira coupling, Sonogashira, Stille coupling, Stille, Hiyama coupling, Hiyama, Negishi coupling, Negishi), the Kumada coupling continues to be employed in many Chemical synthesis, synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. History The first investigations into the ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sonogashira Coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide. :* : aryl or vinyl :* R2: arbitrary :* X: I, Br, Cl or OTf The Sonogashira cross-coupling reaction has been employed in a wide variety of areas, due to its usefulness in the formation of carbon–carbon bonds. The reaction can be carried out under mild conditions, such as at room temperature, in aqueous media, and with a mild base, which has allowed for the use of the Sonogashira cross-coupling reaction in the synthesis of complex molecules. Its applications include pharmaceuticals, natural products, organic materials, and nanomaterials. Specific examples include its use in the synthesis of tazarotene, which is a treatment for psoriasis and acne, and in the preparation of SIB-1508Y, also known as Altinicline, a nicotinic rece ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
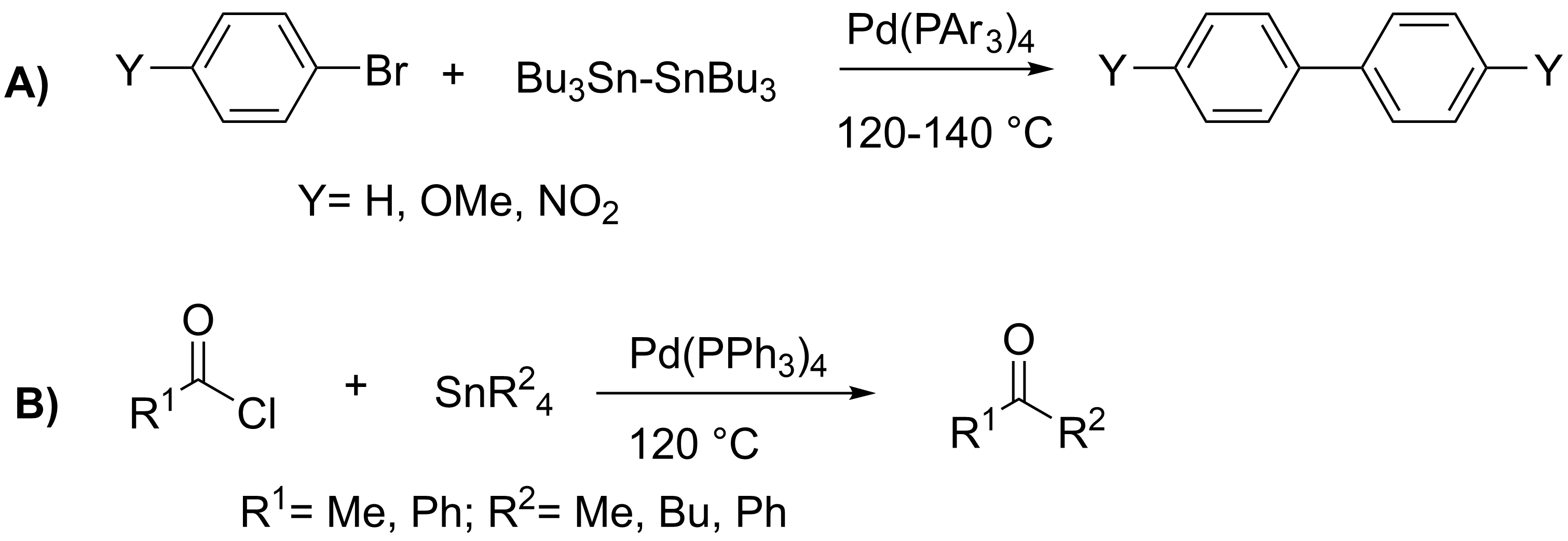
Stille Reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin chemistry, organotin compound (also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many Cross-coupling reaction, palladium-catalyzed coupling reactions.Hartwig, J. F. ''Organotransition Metal Chemistry, from Bonding to Catalysis''; University Science Books: New York, 2010. Stille, J. K. ''Angew. Chem. Int. Ed. Engl.'' 1986, ''25'', 508–524.ReviewFarina, V.; Krishnamurthy, V.; Scott, W. J. ''Org. React.'' 1998, ''50'', 1–652.Review : + \ \ce \ \overbrace^ + \!-\! :*\!,\ : Allyl, alkenyl, aryl, benzyl, acyl :*: halides (Cl, Br, I), pseudohalides (OTf, OPO(OR)2), OAc The R1 group attached to the trialkyltin is normally sp2-hybridized, including vinyl group, vinyl, and aryl groups. These organostannanes are also stable to bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petasis Reaction
The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl- boronic acid to form substituted amines. Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine. In the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery. Reaction scope and synthetic applications The amine is condensed with the carbonyl followed by addition of the boronic acid . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Negishi Coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon–carbon bonds (C–C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc. \begin \\ + \ce \ \ce \ \end :* The leaving group X is usually chloride, bromide, or iodide, but triflate and acetyloxy groups are feasible as well. X = Cl usually leads to slow reactions. :* The organic residue R = alkenyl, aryl, allyl, alkynyl or propargyl. :* The halide X′ in the organozinc compound can be chloride, bromine or iodine and the organic residue R′ is alkenyl, aryl, allyl, alkyl, benzyl, homoallyl, and homopropargyl. :* The metal M in the catalyst is nickel or palladium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suzuki Reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of noble metal catalysis in organic synthesis. This reaction is sometimes telescoped with the related Miyaura borylation; the combination is the Suzuki–Miyaura reaction. It is widely used to synthesize poly olefins, styrenes, and substituted biphenyls. The general scheme for the Suzuki reaction is shown below, where a carbon–carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base. The organoboron species is usually synthesized by hydroboration or carboboration, allowing for rapid generation of molecular complexity. Several reviews have been publ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C ( autoclave) with potassium acetate base and palladium chloride catalysis. This work was an extension of earlier work by Fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coupling Partner
In cross-coupling reactions, the component reagents are called cross-coupling partners or simply coupling partners. These reagents can be further classified according to their nucleophilic vs electrophilic character: :R-X + R'-Y → R-R' + XY Typically the electrophilic coupling partner (R-X) is an aryl halide, but triflates are also used. Nucleophilic coupling (R'-Y) partners are more diverse. In the Suzuki reaction, boronic esters and boronic acids serve as nucleophilic coupling partners. Expanding the scope of coupling partners is a focus methods development in organic synthesis Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ....{{cite journal , title=4-Methoxy-4'-nitrophenyl. Recent Advances In The Stille Biaryl Coupling Reaction and Applications in Complex Natural Produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruthenium Murahashi
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chemicals. Karl Ernst Claus, a Russian scientist of Baltic-German ancestry, discovered the element in 1844 at Kazan State University and named it in honor of Russia. (He used the Latin name ''Ruthenia'', which can have other meanings, but specifically stated that the element was named in honor of his "motherland".) Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemical catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. Ru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |