|
Molybdenum Disulfide
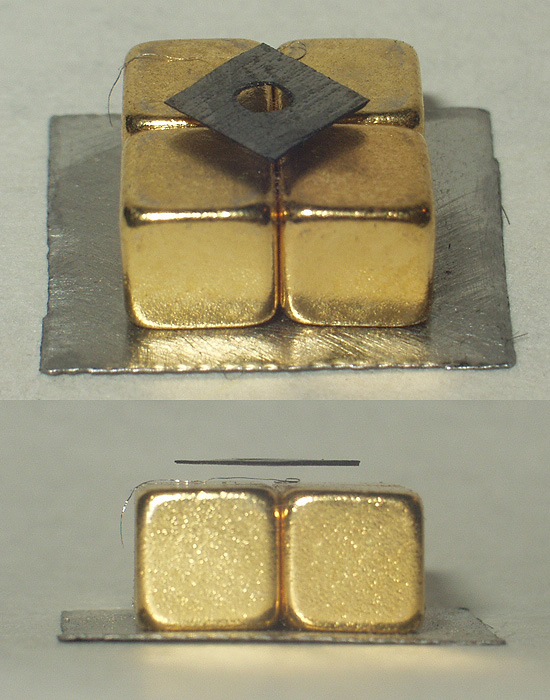
Molybdenum disulfide (or moly) is an inorganic chemistry, inorganic compound composed of molybdenum and sulfur. Its chemical formula is . The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as the mineral molybdenite, the principal ore for molybdenum.Sebenik, Roger F. ''et al''. (2005) "Molybdenum and Molybdenum Compounds", ''Ullmann's Encyclopedia of Chemical Technology''. Wiley-VCH, Weinheim. is relatively unreactive. It is unaffected by dilute acids and oxygen. In appearance and feel, molybdenum disulfide is similar to graphite. It is widely used as a dry lubricant because of its low friction and robustness. Bulk is a Diamagnetism, diamagnetic, indirect bandgap semiconductor similar to silicon, with a bandgap of 1.23 eV. Production is naturally found as either molybdenite, a crystalline mineral, or jordisite, a rare low temperature form of molybdenite. Molybdenite ore is processed by Froth flotation, flotation to give ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqua Regia
Aqua regia (; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar concentration, molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but it turns yellow, orange, or red within seconds from the formation of nitrosyl chloride and nitrogen dioxide. It was so named by alchemy, alchemists because it can dissolve noble metals such as gold and platinum, though not all metals. Preparation and decomposition Upon mixing of concentrated hydrochloric acid and concentrated nitric acid, chemical reactions occur. These reactions result in the volatile products nitrosyl chloride and chlorine gas: : as evidenced by the fuming nature and characteristic yellow color of aqua regia. As the volatile products escape from solution, aqua regia loses its potency. Nitrosyl chloride (NOCl) can further decompose into nitric oxide (NO) and elemental chlorine (): : This dissociation is equilibrium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenite
Molybdenite is a mineral of molybdenum disulfide, Mo S2. Similar in appearance and feel to graphite, molybdenite has a lubricating effect that is a consequence of its layered structure. The atomic structure consists of a sheet of molybdenum atoms sandwiched between sheets of sulfur atoms. The Mo-S bonds are strong, but the interaction between the sulfur atoms at the top and bottom of separate sandwich-like tri-layers is weak, resulting in easy slippage as well as cleavage planes. Molybdenite crystallizes in the hexagonal crystal system as the common polytype 2H and also in the trigonal system as the 3R polytype. Description Occurrence Molybdenite occurs in high temperature hydrothermal ore deposits. Its associated minerals include pyrite, chalcopyrite, quartz, anhydrite, fluorite, and scheelite. Important deposits include the disseminated porphyry molybdenum deposits at Questa, New Mexico and the Henderson and Climax mines in Colorado. Molybdenite also occurs in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum Pentachloride
Molybdenum(V) chloride is the inorganic compound with the empirical formula . This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents. Structure Usually called molybdenum pentachloride, it is in fact partly a dimer with the molecular formula . In the dimer, each molybdenum has local octahedral symmetry and two chlorides bridge between the molybdenum centers. A similar structure is also found for the pentachlorides of W, Nb and Ta. In the gas phase and partly in solution, the dimers partially dissociate to give a monomeric . The monomer is paramagnetic, with one unpaired electron per Mo center, reflecting the fact that the formal oxidation state is +5, leaving one valence electron on the metal center. Preparation and properties is prepared by chlorination of Mo metal but also chlorination of . The unstable hexachloride is not produced in this way. is reduced by acetonitrile to afford an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molly Hill Molybdenite
Molly or mollies may refer to: Animals * Any of the fish in the ''Mollienesia'' subgenus, especially **'' Poecilia sphenops'', short-finned molly **'' Poecilia latipinna'', sailfin molly **'' Poecilia velifera'', Yucatan molly * Fancy molly, domestic hybrids of the above People * Molly (name) or Mollie, a female given name, including a list of persons and characters with the name * Molly Pitcher, one of several American women believed to have helped fight against British forces during the American Revolution * Molly Malone, a mythical 19th-century Irish fishmonger and associated folk song and statue * Molly Mormon, a stereotype of a Latter-day Saints woman * Molly (surname) Dance and theatre * ''Molly'' (musical), a 1973 Broadway musical * Molly dance, a form of English Morris dance Film and television * ''Molly'' (1983 film), an Australian film by Ned Lander * ''Molly'' (1999 film), an American film starring Elisabeth Shue * '' Molly: An American Girl on the Home Fron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indirect Bandgap
In semiconductors, the band gap of a semiconductor can be of two basic types, a direct band gap or an indirect band gap. The minimal-energy state in the conduction band and the maximal-energy state in the valence band are each characterized by a certain crystal momentum (k-vector) in the Brillouin zone. If the k-vectors are different, the material has an "indirect gap". The band gap is called "direct" if the crystal momentum of electrons and holes is the same in both the conduction band and the valence band; an electron can directly emit a photon. In an "indirect" gap, a photon cannot be emitted because the electron must pass through an intermediate state and transfer momentum to the crystal lattice. Examples of direct bandgap materials include hydrogenated amorphous silicon and some III–V materials such as InAs and GaAs. Indirect bandgap materials include crystalline silicon and Ge. Some III–V materials are indirect bandgap as well, for example AlSb. Implications f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetism
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The Permeability (electromagnetism), magnetic permeability of diamagnetic materials is less than the Vacuum_permeability, permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductivity, superconductor acts as a strong diamagnet because it entirely expels any magnetic field from its inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. Types of friction include dry, fluid, lubricated, skin, and internal -- an incomplete list. The study of the processes involved is called tribology, and has a history of more than 2000 years. Friction can have dramatic consequences, as illustrated by the use of friction created by rubbing pieces of wood together to start a fire. Another important consequence of many types of friction can be wear, which may lead to performance degradation or damage to components. It is known that frictional energy losses account for about 20% of the total energy expenditure of the world. As briefly discussed later, there are many different contributors to the retarding force in friction, ranging from asperity deformation to the generation of charges and changes in local structure. When two bodies in contact move relative to each other, due to these variou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Lubricant
Dry lubricants or solid lubricants are materials that, despite being in the solid phase, are able to reduce friction between two surfaces sliding against each other without the need for a liquid oil medium. The two main dry lubricants are graphite and molybdenum disulfide. They offer lubrication at temperatures higher than liquid and oil-based lubricants operate. Dry lubricants are often used in applications such as locks or dry lubricated bearings. Such materials can operate up to 350 °C (662 °F) in oxidizing environments and even higher in reducing / non-oxidizing environments (molybdenum disulfide up to 1100 °C, 2012 °F). The low-friction characteristics of most dry lubricants are attributed to a layered structure on the molecular level with weak bonding between layers. Such layers are able to slide relative to each other with minimal applied force, thus giving them their low friction properties. However, a layered crystal structure alone is not necessar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |