|
Metastudtite
Studtite, chemical formula UO2)O2(H2O)2�2(H2O) or UO4·4(H2O), is a secondary uranium mineral containing peroxide formed by the alpha-radiolysis of water during formation. It occurs as pale yellow to white needle-like crystals often in acicular, white sprays. Studtite was originally described by Vaes in 1947Annales de la Société Géologique de Belgique - 1947 - pp B212 to B226- J.F. Vaes - Six nouveaux minéraux d'urane provenant de Shinkolobwe (Katanga) - from specimens from Shinkolobwe, Katanga Copper Crescent, Katanga (Shaba), Democratic Republic of Congo, and has since been reported from several other localities. The mineral was named for Franz Edward Studt, an English prospector and geologist who was working for the Belgians. When exposed to air studtite converts over a short time to the metastudtite UO4·2(H2O) form. Despite their apparent chemical simplicity, these two uranyl species are the only reported peroxide minerals. They may also be readily formed on the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl Peroxide
Uranyl peroxide or uranium peroxide hydrate (UO4·nH2O) is a pale-yellow, soluble peroxide of uranium. It is found to be present at one stage of the enriched uranium fuel cycle and in yellowcake prepared via the ''in situ'' leaching and resin ion exchange system. This compound, also expressed as UO3·(H2O2)·(H2O), is very similar to uranium trioxide hydrate UO3·''n''H2O. The dissolution behaviour of both compounds are very sensitive to the hydration state (n can vary between 0 and 4). One main characteristic of uranium peroxide is that it consists of small needles with an average AMAD of about 1.1 μm. The uranyl minerals studtite, UO4·4H2O, and metastudtite, UO4·2H2O, are the only minerals discovered to date found to contain peroxide. The product is a light yellow powder. Synthesis In general, uranyl peroxide can be obtained from a solution of uranium (VI) by adding a peroxide, usually hydrogen peroxide solution. The dihydrate is obtained from a boiling solution of uran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corium (nuclear Reactor)
The Three Mile Island reactor 2 after the partial meltdown. Corium, also called fuel-containing material (FCM) or lava-like fuel-containing material (LFCM), is a material that is created in a nuclear reactor core during a nuclear meltdown accident. Resembling lava in consistency, it consists of a mixture of nuclear fuel, fission products, control rods, structural materials from the affected parts of the reactor, products of their chemical reaction with air, water and steam, and, in the event that the reactor vessel is breached, molten concrete from the floor of the reactor room. Composition and formation The heat causing the melting of a reactor may originate from the nuclear chain reaction, but more commonly decay heat of the fission products contained in the fuel rods is the primary heat source. The heat production from radioactive decay drops quickly, as the short half-life isotopes provide most of the heat and radioactive decay, with the curve of decay heat bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide Mineral
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. The minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately. Simple oxides: *XO **Periclase group *** Periclase *** Manganosite **Zincite group *** Zincite *** Bromellite *** Tenorite *** Litharge * ** Cuprite ** Ice * **Hematite group ***Corundum ***Hematite *** Ilmenite * **Rutile group ***Rutile *** Pyrolusite *** Cassiterite ** Baddeleyite ** Uraninite ** Thorianite * **Spinel group *** Spinel *** Gahnite ***Magnetite ***Franklinite *** Chromite ** Chrysoberyl ** Columbite *Hydroxide subgroup: ** Brucite ** Manganite **Romanèchite **Goethite group: *** Diaspore *** Goethite Nickel–Strunz Classification -04- Oxides IMA-CNMNC proposes a new hierarchical scheme (Mills et al., 2009). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geologist
A geologist is a scientist who studies the solid, liquid, and gaseous matter that constitutes Earth and other terrestrial planets, as well as the processes that shape them. Geologists usually study geology, earth science, or geophysics, although backgrounds in physics, chemistry, biology, and other sciences are also useful. Field research (field work) is an important component of geology, although many subdisciplines incorporate laboratory and digitalized work. Geologists can be classified in a larger group of scientists, called geoscientists. Geologists work in the energy and mining sectors searching for natural resources such as petroleum, natural gas, precious and base metals. They are also in the forefront of preventing and mitigating damage from natural hazards and disasters such as earthquakes, volcanoes, tsunamis and landslides. Their studies are used to warn the general public of the occurrence of these events. Geologists are also important contributors to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium(VI) Minerals
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. In nature, uranium is found as uranium-238 (99.2739– ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula . It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing. Structure and bonding The uranyl ion is linear and symmetrical, with both U–O bond lengths of about 180 pm. The bond lengths are indicative of the presence of multiple bonding between the uranium and oxygen atoms. Since uranium(VI) has the electronic configuration of the preceding noble gas, radon, the electrons used in forming the U–O bonds are supplied by the oxygen atoms. The electrons are donated into empty atomic orbitals on the uranium a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Waste
Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment. Radioactive waste is broadly classified into low-level waste (LLW), such as paper, rags, tools, clothing, which contain small amounts of mostly short-lived radioactivity, intermediate-level waste (ILW), which contains higher amounts of radioactivity and requires some shielding, and high-level waste (HLW), which is highly radioactive and hot due to decay heat, so requires cooling and shielding. In nuclear reprocessing plants about 96% of spent nuclear fuel is recycled back into uranium-based and mixed-oxide (MOX) fuels. The residual 4% is minor actinides and fission products the latte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yucca Mountain Nuclear Waste Repository
The Yucca Mountain Nuclear Waste Repository, as designated by the Nuclear Waste Policy Act amendments of 1987, is a proposed deep geological repository storage facility within Yucca Mountain for spent nuclear fuel and other high-level radioactive waste in the United States. The site is on federal land adjacent to the Nevada Test Site in Nye County, Nevada, about northwest of the Las Vegas Valley. The project was approved in 2002 by the 107th United States Congress, but the 112th Congress ended federal funding for the site via amendment to the Department of Defense and Full-Year Continuing Appropriations Act, passed on April 14, 2011, during the Obama Administration. The project has encountered many difficulties and was highly contested by the public, the Western Shoshone peoples, and many politicians. The project also faces strong state and regional opposition. The Government Accountability Office stated that the closure was for political, not technical or safety rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deep Geological Repository
A deep geological repository is a way of storing hazardous or radioactive waste within a stable geologic environment (typically 200–1000 m deep). It entails a combination of waste form, waste package, engineered seals and geology that is suited to provide a high level of long-term isolation and containment without future maintenance. This will prevent any radioactive dangers. A number of mercury, cyanide and arsenic waste repositories are operating worldwide including Canada ( Giant Mine) and Germany (potash mines in Herfa-Neurode and Zielitz) and a number of radioactive waste storages are under construction with the Onkalo in Finland being the most advanced. Principles and background Highly toxic waste that cannot be further recycled must be stored in isolation to avoid contamination of air, ground and underground water. Deep geological repository is a type of long-term storage that isolates waste in geological structures that are expected to be stable for millions of yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
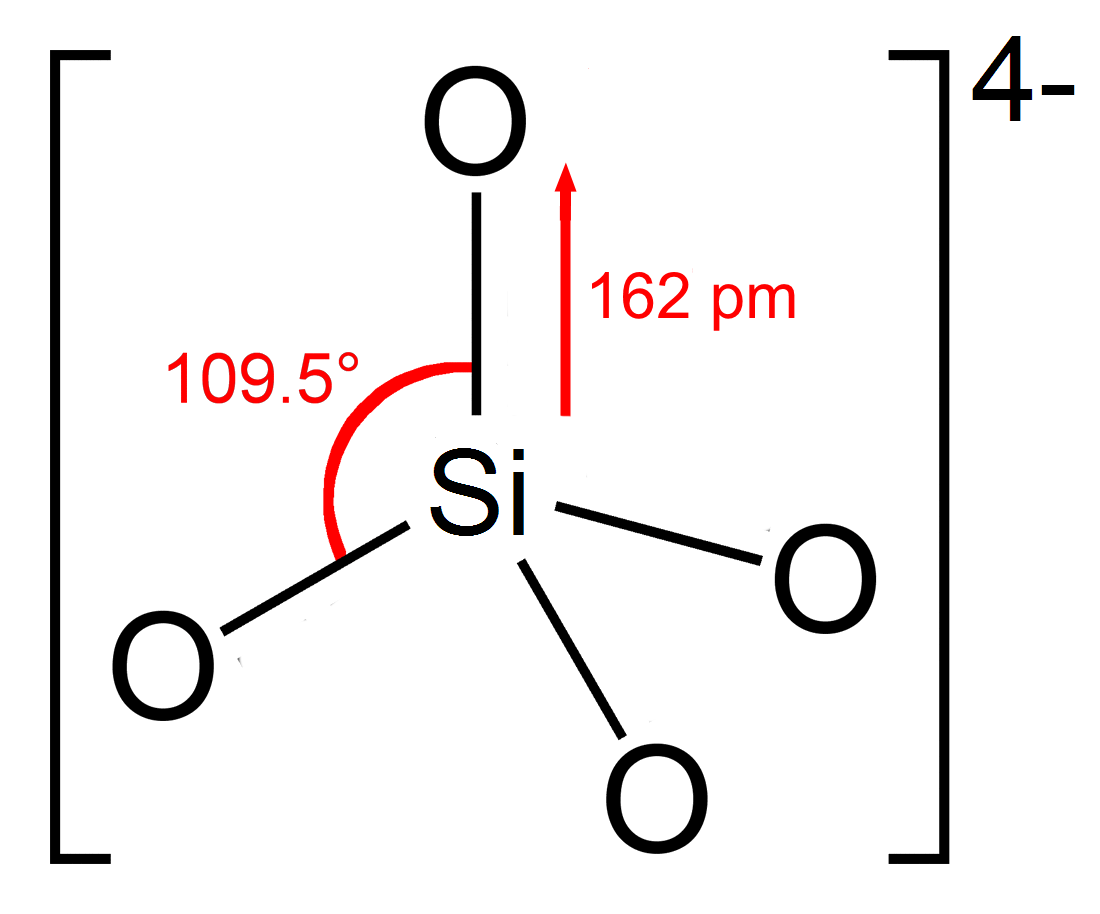
Silicates
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedron whose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Oxide
Uranium oxide is an oxide of the element uranium. The metal uranium forms several oxides: * Uranium dioxide or uranium(IV) oxide (UO2, the mineral uraninite or pitchblende) * Diuranium pentoxide or uranium(V) oxide (U2O5) * Uranium trioxide or uranium(VI) oxide (UO3) * Triuranium octoxide (U3O8), the most stable uranium oxide; yellowcake typically contains 70 to 90 percent triuranium octoxide) * Uranyl peroxide (UO2O2 or UO4) * Amorphous uranium(VI) oxide (''Am''-U2O7) Uranium dioxide is oxidized in contact with oxygen Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ... to form triuranium octoxide. :3 UO2 + O2 → U3O8; at 700 °C (970 K) Preparation 38 During World War II, "Preparation 38" was the codename for uranium oxide used by German scientists. References Oxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chernobyl Disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 nuclear reactor, reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian Soviet Socialist Republic, Ukrainian SSR in the Soviet Union. It is one of only two nuclear energy accidents rated at seven—the maximum severity—on the International Nuclear Event Scale, the other being the 2011 Fukushima nuclear disaster in Japan. The initial emergency response, together with later decontamination of the environment, involved more than Chernobyl liquidators, 500,000 personnel and cost an estimated 18 billion Soviet rouble, roubles—roughly US$68 billion in 2019, adjusted for inflation. The accident occurred during a safety test meant to measure the ability of the steam turbine to power the emergency feedwater pumps of an RBMK, RBMK-type nuclear reactor in the event of a simultaneous loss of external power and major coolant leak. During a pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |