|
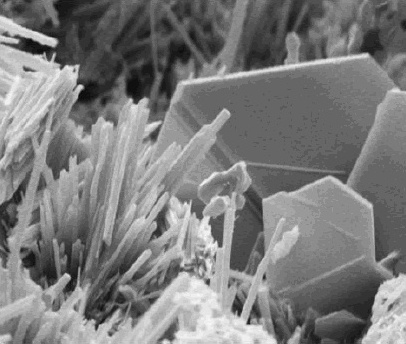
Magnesium Hydroxide
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the Precipitation (chemistry), precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2 OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with calcium hydroxide, lime (Ca(OH)2). A volume of of seawater gives about of Mg(OH)2. Ca(OH)2 ) is far more soluble than Mg(OH)2 ) and dramatically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Common Ion Effect
In chemistry, the common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/ dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other. Examples of the common-ion effect Dissociation of hydrogen sulfide in presence of hydrochloric acid Hydrogen sulfide (H2S) is a weak electrolyte. It is partially ionized when in aqueous solution, therefore there exists an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. Hydroxide ion The hydroxide ion is naturally produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see American and British English spelling differences, spelling differences) is a chemical reaction in which acid and a base (chemistry), base react with an equivalent quantity of each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants. Meaning of "neutralization" In the context of a chemical reaction the term neutralization is used for a reaction between an acid and a base (chemistry), base or alkali. Historically, this reaction was represented as :acid + base (alkali) → salt + water : For example: :HCl + NaOH → NaCl + H2O The statement is still valid as long as it is understood that in an aqueous solution the substances involved are subject to dissociated, dissociation, which changes the ionization state of the substances. The arrow sign, →, is used because ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Santo Domingo - Museo De Ámbar 0669
Santo ('saint' in various languages) may refer to: People * Santo (given name) * Santo (surname) * El Santo, Rodolfo Guzmán Huerta (1917–1984), Mexican wrestler and actor * Bob Santo or Santo, stage name of Ghanaian comedian John Evans Kwadwo Bosompem (1940–2002) * Ferdinand III of Castile (1200–1252) called "''el Santo''" ("the Saint") Places * Santo, Ouest, Haiti, a village * Santō, Shiga, Japan, a town * Santo, Texas, United States, an unincorporated community * Basilica of Saint Anthony of Padua, Italy, known locally as ''il Santo'' *Espiritu Santo, the largest island of Vanuatu, nicknamed Santo **Luganville, known locally as Santo Arts and entertainment *Santo (art), a wooden or ivory statue depicting a holy figure * ''Santo'' (EP), by Alonso Brito, 2008 * "Santo" (song), by Christina Aguilera, 2022 *"Santo", a song by Ely Buendia * ''Il Santo'' (novel), Antonio Fogazzaro, 1905 See also * * *Los Santos (other) *Santos (other) *Santa (disambigu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Global Library Of Women's Medicine
The ''Global Library of Women's Medicine'' is a free and publicly available resource of clinical information on women's health launched on 20 November 2008. Its purpose is to provide expert support to obstetricians, gynecologists, and reproductive health professionals. __TOC__ History The site is contributed to by over 750 specialists and its main feature is a large and continually growing library of specially commissioned chapters on most aspects of women's medicine, constantly reviewed and updated. This has recently been supported by the introduction of an online Learning Assessment option, providing Certificates of Study Completion plus Continuing Professional Development awards (from FIGO – International Federation of Gynaecology and Obstetrics) for those who undertake this assessment successfully The site also includes an extensive section on Safer Motherhood providing practical guidance for nurses, midwives and other healthcare workers and it offers a free Safer Motherh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypermagnesemia
Hypermagnesemia is an electrolyte disorder in which there is a high level of magnesium in the blood. Symptoms include weakness, confusion, decreased breathing rate, and decreased reflexes. Hypermagnesemia can greatly increase the chances of adverse cardiovascular events. Complications may include low blood pressure and cardiac arrest. It is typically caused by kidney failure or is treatment-induced such as from antacids or supplements that contain magnesium. Less common causes include tumor lysis syndrome, seizures, and prolonged ischemia. Diagnosis is based on a blood level of magnesium greater than 1.1 mmol/L (2.6 mg/dL). It is severe if levels are greater than 2.9 mmol/L (7 mg/dL). Specific electrocardiogram (ECG) changes may be present. Treatment involves stopping the magnesium a person is getting. Treatment when levels are very high include calcium chloride, intravenous normal saline with furosemide, and hemodialysis. Hypermagnesemia is uncommon. Rates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degasification
Degassing, also known as degasification, is the removal of dissolved gases from liquids, especially water or aqueous solutions. There are numerous methods for removing gases from liquids. Gases are removed for various reasons. Chemists remove gases from solvents when the compounds they are working on are possibly air- or oxygen-sensitive (air-free technique), or when bubble formation at solid-liquid interfaces becomes a problem. The formation of gas bubbles when a liquid is frozen can also be undesirable, necessitating degassing beforehand. Pressure reduction The solubility of gas obeys Henry's law, that is, the amount of a dissolved gas in a liquid is proportional to its partial pressure. Therefore, placing a solution under reduced pressure makes the dissolved gas less soluble. Sonication and stirring under reduced pressure can usually enhance the efficiency. This technique is often referred to as ''vacuum degasification''. Specialized vacuum chambers, called vacuum degassers, ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation Exchange Membrane
An ion-exchange membrane is a semi-permeable membrane that transports certain dissolved ions, while blocking other ions or neutral molecules. Ion-exchange membranes are therefore electrically conductive. They are often used in desalination and chemical recovery applications, moving ions from one solution to another with little passage of water. Important examples of ion-exchange membranes include the proton-exchange membranes, that transport cations, and the anion exchange membranes used in certain alkaline fuel cells to transport anions. Structure and composition An ion-exchange membrane is generally made of organic or inorganic polymer with charged (ionic) side groups, such as ion-exchange resins. Anion-exchange membranes contain fixed cationic groups with predominantly mobile anions; because anions are the majority species, most of the conductivity is due to anion transport. The reverse holds for cation-exchange membranes. The so-called heterogeneous ion-exchang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ca(OH)2
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide. Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Solubility Calcium hydroxide is moderately soluble in water, as seen for many dihydroxides. Its solubility increases from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. Its solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NaOH
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures and at high concentrations may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper, textiles, drinking w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Trichloride
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula . This yellow, oily, and explosive liquid is most commonly encountered as a product of chemical reactions between ammonia-derivatives and chlorine (for example, in swimming pools). Alongside monochloramine and dichloramine, trichloramine is responsible for the distinctive 'chlorine smell' associated with swimming pools, where the compound is readily formed as a product from hypochlorous acid reacting with ammonia and other nitrogenous substances in the water, such as urea from urine. Preparation and occurrence The compound is generated by treatment of ammonium chloride with calcium hypochlorite. When prepared in an aqueous-dichloromethane mixture, the trichloramine is extracted into the nonaqueous phase. Intermediates in this conversion include monochloramine and dichloramine, and , respectively. Nitrogen trichloride, trademarked as Agene, was at one time used to bleach flour, but th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |