|
Lithium Borate
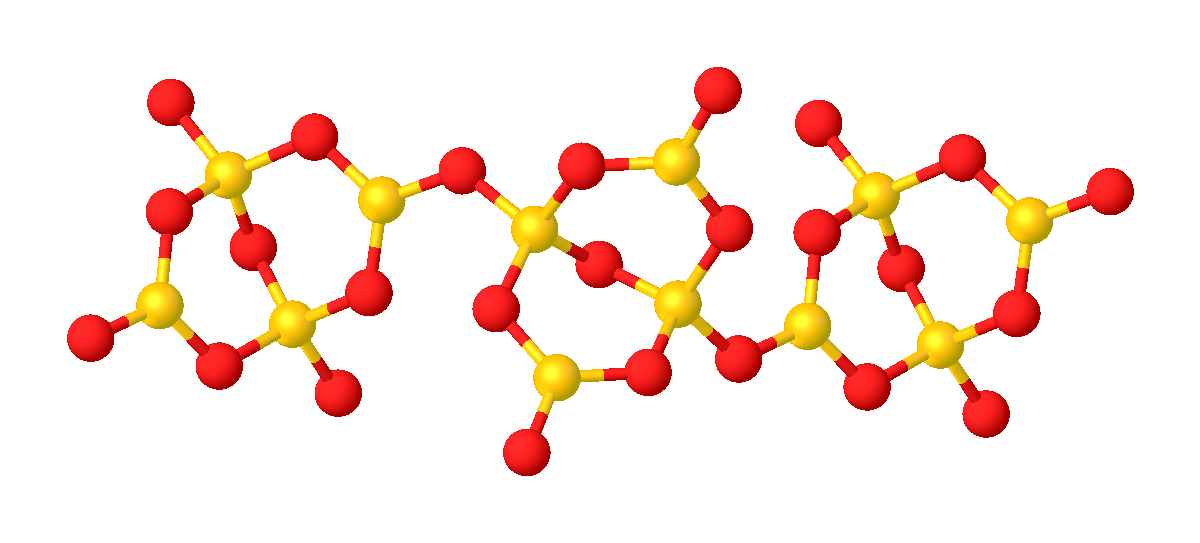
Lithium borate, also known as lithium tetraborate, dilithium tetraborate or boron lithium oxide is an inorganic compound with the formula Li2B4O7. A colorless solid, lithium borate is used in making glasses and ceramics. It is not to be confused with B8Li2O13, also called lithium borate. Structure Its structure consists of a polymeric borate backbone. The Li+ centers are bound to four and five oxygen ligands. Boron centers are trigonal and tetrahedral. Lithium borate can be used in the laboratory as LB buffer for gel electrophoresis of DNA and RNA. It is also used in the borax fusion method to vitrify mineral powder specimens for analysis by WDXRF spectroscopy.Ron Jenkins, ''X-Ray Fluorescence Spectrometry, Second Edition'', J. Wiley & Sons Inc., 1999, , p 146-7. See also *LB buffer LB buffer, also known as lithium borate buffer, is a buffer solution used in agarose electrophoresis, typically for the separation of nucleic acids such as DNA and RNA. It is made up of Lithium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were fired clay bricks used for building house walls and other structures. Other pottery objects such as pots, vessels, vases and figurines were made from clay, either by itself or mixed with other materials like silica, hardened by sintering in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial, and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as semiconductors. The word '' ceramic'' comes from the Ancient Greek word (), meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LB Buffer
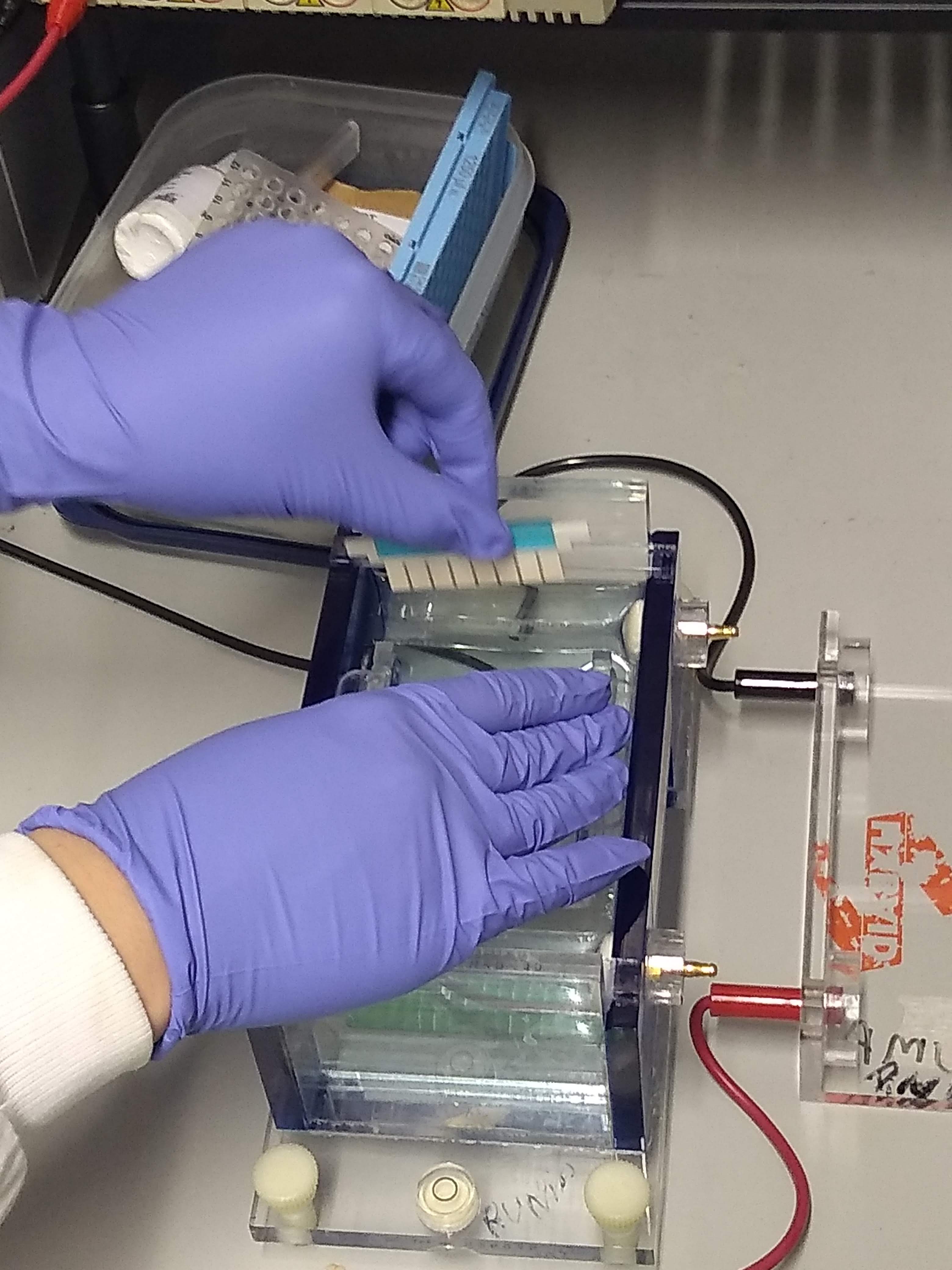
LB buffer, also known as lithium borate buffer, is a buffer solution used in agarose electrophoresis, typically for the separation of nucleic acids such as DNA and RNA. It is made up of Lithium borate (lithium hydroxide monohydrate and boric acid). LB(R) is a registered (USPTO) trademark of Faster Better Media LLC, which owns US patent 7,163,610 covering low-conductance lithium borate polynucleotide electrophoresis. Lithium Borate buffer has a lower conductivity, produces crisper resolution, and can be run at higher speeds than can gels made from TBE or TAE (5-50 V/cm as compared to 5-10 V/cm). At a given voltage, the heat generation and thus the gel temperature is much lower than with TBE/TAE buffers, therefore the voltage can be increased to speed up electrophoresis so that a gel run takes only a fraction of the usual time. Downstream applications, such as isolation of DNA from a gel slice or Southern blot analysis, work as expected with lithium boric acid gels. Brody, J.R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Electrophoresis
Gel electrophoresis is an electrophoresis method for separation and analysis of biomacromolecules (DNA, RNA, proteins, etc.) and their fragments, based on their size and charge through a gel. It is used in clinical chemistry to separate proteins by charge or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments, or to separate proteins by charge. Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a gel matrix of agarose, polyacrylamide, or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving. Proteins are separated by the charge in agarose because the pores of the gel are too large to sieve proteins. Gel electrophoresi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitrification
Vitrification (, via French ') is the full or partial transformation of a substance into a glass, that is to say, a non- crystalline or amorphous solid. Glasses differ from liquids structurally and glasses possess a higher degree of connectivity with the same Hausdorff dimensionality of bonds as crystals: dimH = 3. In the production of ceramics, vitrification is responsible for their impermeability to water. Vitrification is usually achieved by heating materials until they liquify, then cooling the liquid, often rapidly, so that it passes through the glass transition to form a glassy solid. Certain chemical reactions also result in glasses. In terms of chemistry, vitrification is characteristic for amorphous materials or disordered systems and occurs when bonding between elementary particles (atoms, molecules, forming blocks) becomes higher than a certain threshold value. Thermal fluctuations break the bonds; therefore, the lower the temperature, the higher the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Fluorescence
X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and analytical chemistry, chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings. Underlying physics When materials are exposed to short-wavelength X-rays or to gamma rays, ionization of their component atoms may take place. Ionization consists of the ejection of one or more electrons from the atom, and may occur if the atom is exposed to radiation with an energy greater than its ionization energy. X-rays and gamma rays can be energetic enough to expel tightly held electrons from the inner atomic orbital, orbitals of the atom. The removal of an electron in this way makes the electronic structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Metaborate
Lithium metaborate is a chemical compound of lithium, boron, and oxygen with elemental formula . It is often encountered as a hydrate, , where ''n'' is usually 2 or 4. However, these formulas do not describe the actual structure of the solids. Lithium metaborate is one of the borates, a large family of salts (ionic compounds) with anions consisting of boron, oxygen, and hydrogen. Structure Lithium metaborate has several crystal forms. The α form consists of infinite chains of trigonal planar metaborate anions . The γ form is stable at 15 kbar and 950 °C. It has a polymeric cation consisting of a tridimensional regular array of tetrahedra sharing oxygen vertices, alernating with lithium cations, each also surrounded by four oxygen atoms. The B-O distances are 148.3 pm, the Li-O distances are 196 pm. Lithium metaborate forms glass relatively easily, and consists of approximately 40% tetrahedral borate anions, and 60% trigonal planar boron. The ratio of tetrahedral to tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borates
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt (chemistry), salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of such anions, such as trimethyl borate . Natural occurrence Borate ions occur, alone or with other anions, in many borate mineral, borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, contributing to the absorption of low-frequency sound in seawater. Common borate salts include sodium metaborate (NaBO2) and borax. Borax is soluble in water, so mineral deposits only occur in places with very low rainfall. Extensive deposits were found in Death Valley and shipped with twenty-mule teams from 1883 to 1889. In 1925, deposits were found at Boron, California, Boron, California on the edge of the Mojave Desert. The Atacama Desert in Chile also contains mineab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |