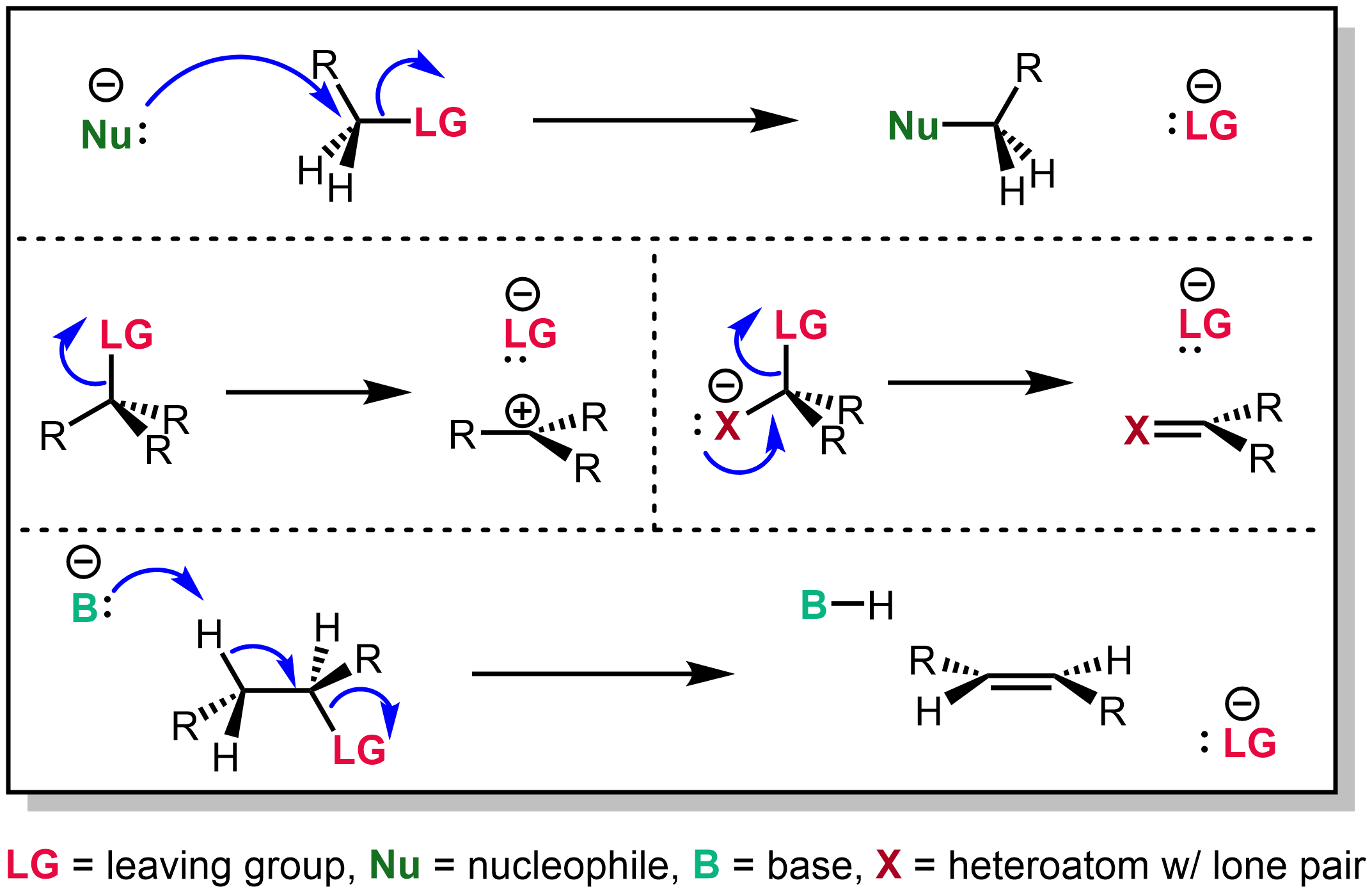
|
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Groups-3-types-of-reactions
Leaving or Leavin' may refer to: Film, theatre and television * ''Leaving'' (TV series), a 1984–1985 UK series featuring Keith Barron and Susan Hampshire * ''Leaving'' (1997 film), a Japanese film starring Kotomi Kyono * ''Leaving'' (play), a 2007 play by Václav Havel ** ''Leaving'' (2011 film), a Czech film directed by Václav Havel and based on his play * ''Leaving'' (2009 film), a French film by Catherine Corsini * ''Leaving'', a 2012 UK television series featuring Linzey Cocker * ''The Leaving'', a 2018 Philippine animated film * "Leaving" (''Watching''), a 1987 television episode Music Albums * ''Leaving'' (album), a 1976 album by Richard Beirach and Jeremy Steig * ''Leavin (album), a 2006 album by Natalie Cole EP * ''Leaving'' (EP), a 2013 EP by Skrillex whose title track is "Leaving" Songs * "Leavin' " (Tony! Toni! Toné! song), 1994 * "Leaving", a 2001 song by The Starting Line from '' With Hopes of Starting Over...'' * "Leavin' " (Jesse McCar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elementary Step
In chemistry, a reaction step of a chemical reaction is defined as: ''"An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate (or, for the first step, the reactants) is converted into the next reaction intermediate (or, for the last step, the products) in the sequence of intermediates between reactants and products"''. – Gold Book
The International Union of Pure and Applied Chemistry (IUPAC) publishes many books which contain its complete list of definitions. The definitions are divided initially into seven IUPAC ...
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State Theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ''H''‡, also written Δ‡''H''ɵ), the standard entropy of activation (Δ''S''‡ or Δ‡''S''ɵ), and the standard Gibbs energy of activation (Δ''G''‡ or Δ‡''G''ɵ) for a particular reaction if its rate constant has been experimentally determined (the ‡ notation refers to the value of interest ''at the transition state''; Δ''H''‡ is the difference between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit time. Reaction rates can vary dramatically. For example, the oxidative rusting of iron under Earth's atmosphere is a slow reaction that can take many years, but the combustion of cellulose in a fire is a reaction that takes place in fractions of a second. For most reactions, the rate decreases as the reaction proceeds. A reaction's rate can be determined by measuring the changes in concentration over time. Chemical kinetics is the part of physical chemistry that concerns how rates of chemical reactions are measured and predicted, and how reaction-rate data can be used to deduce probable reaction mechanisms. The concepts of chemical kinetics are applied in many disciplines, such as chemical engineering, enzymology and environmental e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dication
A dication is any cation, of general formula X2+, formed by the removal of two electrons from a neutral species. Diatomic dications corresponding to stable neutral species (e.g. formed by removal of two electrons from H2) often decay quickly into two singly charged particles (H+), due to the loss of electrons in bonding molecular orbitals. Energy levels of diatomic dications can be studied with good resolution by measuring the yield of pairs of zero-kinetic-energy electrons from double photoionization of a molecule as a function of the photoionizing wavelength (threshold photoelectrons coincidence spectroscopy – TPEsCO). The dication is kinetically stable. An example of a stable diatomic dication which is not formed by oxidation of a neutral diatomic molecule is the dimercury dication . An example of a polyatomic dication is , formed by oxidation of S8 and unstable with respect to further oxidation over time to form SO2. Many organic dications can be detected in mass spectro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleofuge
In chemistry, a nucleofuge () is a leaving group which retains the lone pair of electrons from its previous bond with another species. For example, in the SN2 mechanism, a nucleophile attacks an organic compound containing the nucleofuge (the bromo group) which simultaneously breaks the bond with the nucleofuge. After a reaction nucleofuges may contain either a negative or a neutral charge; this is governed by the nature of the specific reaction. The word 'nucleofuge' is commonly found in older literature, but its use is less common in current literature in which the term ''leaving group'' dominates. See also * Electrofuge *Nucleophile *Electrophile In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ... References * Organic chemistry {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; that is, the timescale over which it begins to degrade. Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibrium in which individual atoms or molecules change form, but their overall number in a particular form is conserved. This type of chemical thermodynamic equilibrium will persist indefinitely unless the system is changed. Chemical systems might undergo changes in the phase of matter or a set of chemical reactions. State A is said to be more thermodynamically stable than state B if the Gibbs free energy of the change from A to B is positive. Versus reactivity Thermodynamic stability applies to a particular system. The reactivity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |