|
Large T Antigen
The large tumor antigen (also called the large T-antigen and abbreviated LTag or LT) is a protein encoded in the genomes of polyomaviruses, which are small double-stranded DNA viruses. LTag is gene expression, expressed early in the infectious cycle and is essential gene, essential for viral proliferation. Containing four conserved gene, well-conserved protein domains as well as several intrinsically disordered regions, LTag is a fairly large multifunctional protein; in most polyomaviruses, it ranges from around 600-800 amino acids in length. LTag has two primary functions, both related to replication of the viral genome: it unwinds the virus's DNA to prepare it for replication, and it interacts with proteins in the host cell to dysregulate the cell cycle so that the host's DNA replication machinery can be used to replicate the virus's genome. Some polyomavirus LTag proteins - most notably the well-studied SV40 large T antigen, SV40 large tumor antigen from the SV40 virus - are oncop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
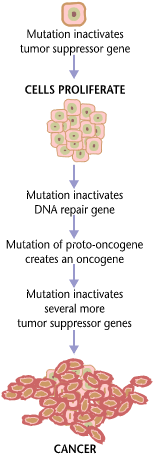
Neoplastic Transformation
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''Asn, followed by anything but Pro, followed by either Ser or Thr, followed by anything but Pro residue''. Overview When a sequence motif appears in the exon of a gene, it may encode the " structural motif" of a protein; that is a stereotypical element of the overall structure of the protein. Nevertheless, motifs need not be associated with a distinctive secondary structure. " Noncoding" sequences are not translated into proteins, and nucleic acids with such motifs need not deviate from the typical shape (e.g. the "B-form" DNA double helix). Outside of gene exons, there exist regulatory sequence motifs and motifs within the " junk", such as satellite DNA. Some of these are believed to affect the shape of nucleic acids (see for example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hsc70 Heat-shock Proteins
Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the ''HSPA8'' gene on chromosome 11. As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging. Structure This gene encodes a 70kDa heat shock protein which is a member of the heat shock protein 70 (Hsp70) family. As a Hsp70 protein, it has a C-terminal protein substrate-binding domain and an N-terminal ATP-binding domain. The substrate-binding domain consists of two subdomains, a two-layered β-sandwich subdomain (SBDβ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone Protein
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DnaJ
In molecular biology, chaperone DnaJ, also known as Hsp40 (heat shock protein 40 kDa), is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans. Function Molecular chaperones are a diverse family of proteins that function to protect proteins from irreversible Protein aggregation, aggregation during synthesis and in times of cellular stress. The bacterial molecular chaperone Hsp70, DnaK is an enzyme that couples cycles of Adenosine triphosphate, ATP binding, hydrolysis, and Adenosine diphosphate, ADP release by an N-terminal ATP-hydrolyzing domain to cycles of sequestration and release of unfolded proteins by a C-terminal substrate binding domain. Dimeric GrpE is the co-chaperone for DnaK, and acts as a nucleotide exchange factor, stimulating the rate of ADP release 5000-fold. DnaK is itself a weak ATPase; ATP hydrolysis by DnaK is stimulated by its interaction with another co-chaperone, DnaJ. Thus the co-chaperones DnaJ and GrpE ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
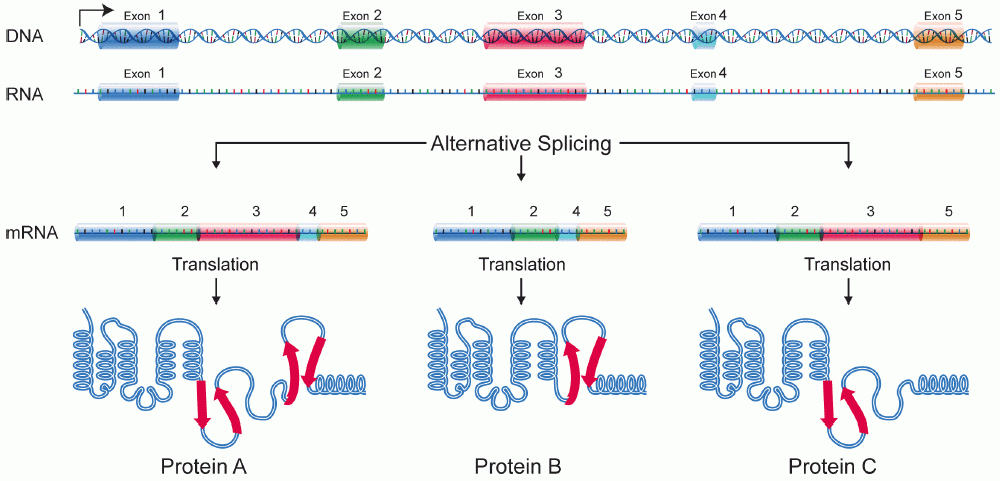
Alternative Splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative RNA splicing, splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene may be included within or excluded from the final RNA product of the gene. This means the exons are joined in different combinations, leading to different splice variants. In the case of protein-coding genes, the proteins translated from these splice variants may contain differences in their amino acid sequence and in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most of the observed splice variants ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylated
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols: : This equation can be written in several ways that are nearly equivalent that describe the behaviors of various protonated states of ATP, ADP, and the phosphorylated product. As is clear from the equation, a phosphate group per se is not transferred, but a phosphoryl group (PO3-). Phosphoryl is an electrophile. This process and its inverse, dephosphorylation, are common in biology. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. During respiration Phosphorylation is essential to the processes of both anaerobic and aerobic respiration, which involve the production of adenosine triphosphate (ATP), the "high-energy" exchang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, ATP hydrolase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life. Some such enzymes are integral membrane proteins (anchored within biological membranes), and move solutes across the membrane, typically against their concentration gradient. These are called transmembrane ATPases. Functions Transmembrane ATPases import metabolites necessary for cell metabolism and export toxins, wastes, and solutes that can hinder cellular processes. An important example is the sodium-potassium pump (Na+/K+ATPase) that maintains the cell membrane potential. Another example is the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AAA Protein
AAA (ATPases Associated with diverse cellular Activities) proteins (speak: triple-A ATPases) are a large group of protein family sharing a common conserved module of approximately 230 amino acid residues. This is a large, functionally diverse protein family belonging to the AAA+ protein superfamily of ring-shaped P-loop NTPases, which exert their activity through the energy-dependent remodeling or translocation of macromolecules. AAA proteins couple chemical energy provided by ATP hydrolysis to conformational changes which are transduced into mechanical force exerted on a macromolecular substrate. AAA proteins are functionally and organizationally diverse, and vary in activity, stability, and mechanism. Members of the AAA family are found in all organisms and they are essential for many cellular functions. They are involved in processes such as DNA replication, protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |