|
GRP94
Heat shock protein 90kDa beta member 1 (HSP90B1), known also as endoplasmin, gp96, grp94, or ERp99, is a chaperone protein that in humans is encoded by the ''HSP90B1'' gene. HSP90B1 is an HSP90 paralogue that is found in the endoplasmic reticulum. It plays critical roles in folding proteins in the secretory pathway such as Toll-like receptors and integrins., It has been implicated as an essential immune chaperone to regulate both innate and adaptive immunity. Tumor-derived HSP90B1 (vitespen) has entered clinical trials for cancer immunotherapy Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the basic research, fundamental research of cancer im .... grp94 has been shown to be a target for treatment of a plethora of diseases such as glaucoma, multiple myeloma, and metastatic cancer. grp94 includes 5 distinct amino acids in its primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone Protein
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hsp90
Hsp90 (heat shock protein 90) is a chaperone (protein), chaperone protein that assists other proteins to protein folding, fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs. Heat shock proteins, as a class, are among the most highly expressed cell (biology), cellular proteins across all species. As their name implies, heat shock proteins protect cells when stressed by elevated temperatures. They account for 1–2% of total protein in unstressed cells. However, when cells are heated, the fraction of heat shock proteins increases to 4–6% of cellular proteins. Heat shock protein 90 (Hsp90) is one of the most common of the heat-related proteins. The "90" comes from the fact that it has a mass of roughly 90 Atomic mass unit, kilodaltons. A 90 kDa protein is considered fairly large for a non-fibrou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Chaperones
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermediat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and non-coding genes. During gene expression (the synthesis of Gene product, RNA or protein from a gene), DNA is first transcription (biology), copied into RNA. RNA can be non-coding RNA, directly functional or be the intermediate protein biosynthesis, template for the synthesis of a protein. The transmission of genes to an organism's offspring, is the basis of the inheritance of phenotypic traits from one generation to the next. These genes make up different DNA sequences, together called a genotype, that is specific to every given individual, within the gene pool of the population (biology), population of a given species. The genotype, along with environmental and developmental factors, ultimately determines the phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology (biology)
In biology, homology is similarity in anatomical structures or genes between organisms of different taxa due to shared ancestry, ''regardless'' of current functional differences. Evolutionary biology explains homologous structures as retained heredity from a common descent, common ancestor after having been subjected to adaptation (biology), adaptive modifications for different purposes as the result of natural selection. The term was first applied to biology in a non-evolutionary context by the anatomist Richard Owen in 1843. Homology was later explained by Charles Darwin's theory of evolution in 1859, but had been observed before this from Aristotle's biology onwards, and it was explicitly analysed by Pierre Belon in 1555. A common example of homologous structures is the forelimbs of vertebrates, where the bat wing development, wings of bats and origin of avian flight, birds, the arms of primates, the front flipper (anatomy), flippers of whales, and the forelegs of quadrupedalis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toll-like Receptors
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane protein, single-spanning receptor (biochemistry), receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from Microorganism, microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in Intracellular receptor, intracellular Vesicle (biology and chemistry), vesicles (because they are sensors of nucleic acids). TLRs received their name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrins
Integrins are transmembrane receptors that help cell–cell and cell– extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Innate Immunity
The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates (the other being the adaptive immune system). The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants, fungi, prokaryotes, and invertebrates (see Beyond vertebrates).. The major functions of the innate immune system are to: * recruit immune cells to infection sites by producing chemical factors, including chemical mediators called cytokines * activate the complement cascade to identify bacteria, activate cells, and promote clearance of antibody complexes or dead cells * identify and remove foreign substances present in organs, tissues, blood and lymph, by specialized white blood cells * activate the adaptive immune system through antigen presentation * act as a physical and chemical barrier to infectious agents; via physical measures such as skin and mucus, and chemical measures such as clotting factors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adaptive Immunity
The adaptive immune system (AIS), also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized cells, organs, and processes that eliminate pathogens specifically. The acquired immune system is one of the two main immunity strategies found in vertebrates (the other being the innate immune system). Like the innate system, the adaptive immune system includes both humoral immunity components and cell-mediated immunity components and destroys invading pathogens. Unlike the innate immune system, which is pre-programmed to react to common broad categories of pathogen, the adaptive immune system is highly specific to each particular pathogen the body has encountered. Adaptive immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to future encounters with that pathogen. Antibodies are a critical part of the adaptive immune system. Adaptive im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
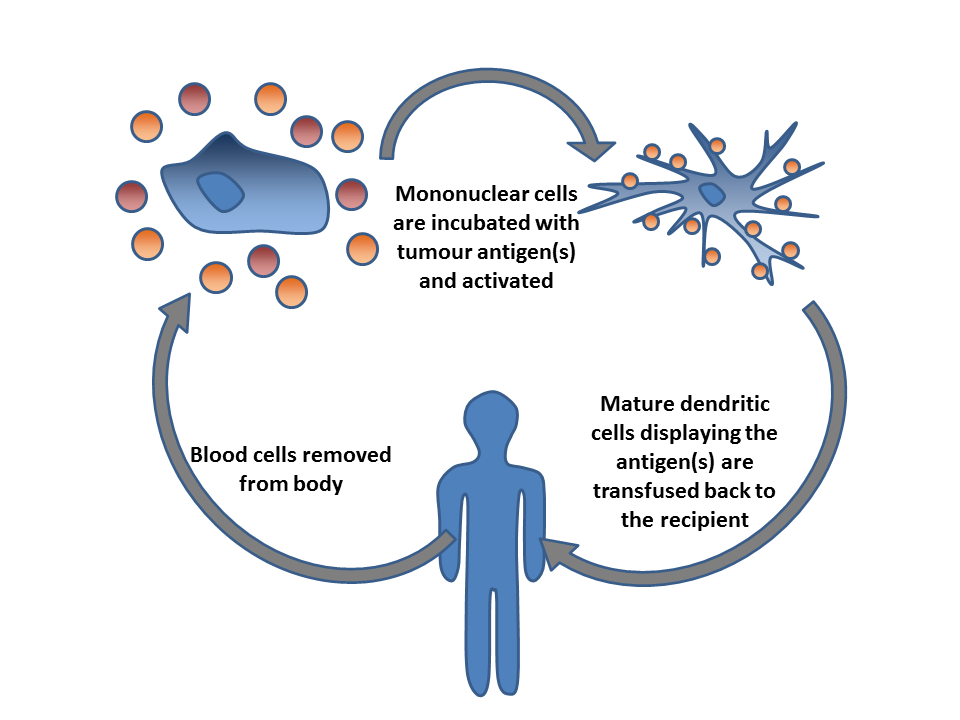
Cancer Immunotherapy
Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the basic research, fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology. Cancer immunotherapy exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can bind to antibody proteins or T-cell receptors, triggering an immune system response. The tumor antigens are often proteins or other macromolecules (e.g., carbohydrates). Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill. The clinical success of cancer immunotherapy is highly variable between different forms of cancer; for instance, certain subtypes of gastric cancer react well to the approach whereas immunothera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |