|
Free Entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu–Planck potentials (or functions), or (rarely) free information. In statistical mechanics, free entropies frequently appear as the logarithm of a partition function. The Onsager reciprocal relations in particular, are developed in terms of entropic potentials. In mathematics, free entropy means something quite different: it is a generalization of entropy defined in the subject of free probability. A free entropy is generated by a Legendre transformation of the entropy. The different potentials correspond to different constraints to which the system may be subjected. Examples The most common examples are: where ::S is entropy ::\Phi is the Massieu potential ::\Xi is the Planck potential ::U is internal energy ::T is temperature ::P is pressure ::V is volume ::A is Helmholtz free energy ::G is Gibbs free energy ::N_i is number of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volume (thermodynamics)
In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law. The physical volume of a system may or may not coincide with a control volume used to analyze the system. Overview The volume of a thermodynamic system typically refers to the volume of the working fluid, such as, for example, the fluid within a piston. Changes to this volume may be made through an application of work, or may be used to produce work. An isochoric process however operates at a constant-volume, thus no work can be produced. Many other thermodynamic processes will result in a change in volume. A polytropic process, in particular, causes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intensive And Extensive Properties
Physical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. According to IUPAC, an intensive quantity is one whose magnitude is independent of the size of the system, whereas an extensive quantity is one whose magnitude is additive for subsystems. The terms ''intensive and extensive quantities'' were introduced into physics by German writer Georg Helm in 1898, and by American physicist and chemist Richard C. Tolman in 1917. An intensive property does not depend on the system size or the amount of material in the system. It is not necessarily homogeneously distributed in space; it can vary from place to place in a body of matter and radiation. Examples of intensive properties include temperature, ''T''; refractive index, ''n''; density, ''ρ''; and hardness, ''η''. By contrast, extensive properties such as the mass, volume and entropy of syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Potentials
A thermodynamic potential (or more accurately, a thermodynamic potential energy)ISO/IEC 80000-5, Quantities an units, Part 5 - Thermodynamics, item 5-20.4 Helmholtz energy, Helmholtz functionISO/IEC 80000-5, Quantities an units, Part 5 - Thermodynamics, item 5-20.5, Gibbs energy, Gibbs function is a scalar quantity used to represent the thermodynamic state of a system. The concept of thermodynamic potentials was introduced by Pierre Duhem in 1886. Josiah Willard Gibbs in his papers used the term ''fundamental functions''. One main thermodynamic potential that has a physical interpretation is the internal energy . It is the energy of configuration of a given system of conservative forces (that is why it is called potential) and only has meaning with respect to a defined set of references (or data). Expressions for all other thermodynamic energy potentials are derivable via Legendre transforms from an expression for . In thermodynamics, external forces, such as gravity, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
François Massieu
François Jacques Dominique Massieu (4 August 1832 – 5 February 1896) was a French thermodynamics engineer noted for his two 1869 characteristic functions, each of which known as a Massieu function (the first of which sometimes called free entropy), as cited by American engineer Willard Gibbs in his 1876 ''On the Equilibrium of Heterogeneous Substances In the history of thermodynamics, ''On the Equilibrium of Heterogeneous Substances'' is a 300-page paper written by American chemical physicist Willard Gibbs. It is one of the founding papers in thermodynamics, along with German physicist Hermann ...''. References External links *Nivoit, E. (1897). �Notice of the Life and Work of Mr. Massieu Inspector General of Mines” (French → English), Annales des Mines, 9th Series, Vol. 11. {{DEFAULTSORT:Massieu, Francois 1832 births 1896 deaths Thermodynamicists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planck
Max Karl Ernst Ludwig Planck (, ; 23 April 1858 – 4 October 1947) was a German theoretical physicist whose discovery of energy quanta won him the Nobel Prize in Physics in 1918. Planck made many substantial contributions to theoretical physics, but his fame as a physicist rests primarily on his role as the originator of quantum theory, which revolutionized human understanding of atomic and subatomic processes. In 1948, the German scientific institution Kaiser Wilhelm Society (of which Planck was twice president) was renamed Max Planck Society (MPG). The MPG now includes 83 institutions representing a wide range of scientific directions. Life and career Planck came from a traditional, intellectual family. His paternal great-grandfather and grandfather were both theology professors in Göttingen; his father was a law professor at the University of Kiel and Munich. One of his uncles was also a judge. Planck was born in 1858 in Kiel, Holstein, to Johann Julius Wilhelm Pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Species
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. These energy levels determine the way the chemical species will interact with others (engaging in chemical bonds, etc.). The species can be an atom, molecule, ion, or radical, and it has a specific chemical name and chemical formula. The term is also applied to a set of chemically identical atomic or molecular structural units in a solid array. In supramolecular chemistry, chemical species are those supramolecular structures whose interactions and associations are brought about via intermolecular bonding and debonding actions, and function to form the basis of this branch of chemistry. For instance: * The chemical species argon is an atomic species of formula Ar; * dioxygen and ozone are different molecular species, of respective formulas O and O; * chloride is an ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
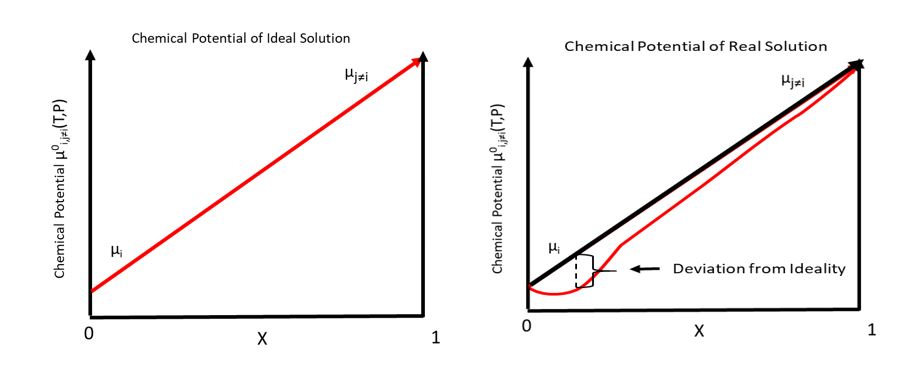
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Number
The particle number (or number of particles) of a thermodynamic system, conventionally indicated with the letter ''N'', is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle number is a dimensionless quantity. It is an extensive parameter, as it is directly proportional to the size of the system under consideration, and thus meaningful only for closed systems. A constituent particle is one that cannot be broken into smaller pieces at the scale of energy ''k·T'' involved in the process (where ''k'' is the Boltzmann constant and ''T'' is the temperature). For example, for a thermodynamic system consisting of a piston containing water vapour, the particle number is the number of water molecules in the system. The meaning of constituent particle, and thereby of particle number, is thus temperature-dependent. Determining the parti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces. The Gibbs energy is the thermodynamic potential that is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature (isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz free energy is often used, since explosive reactions by their nature induce pressure changes. It is also frequently used to define fundamental equations of state of pure substances. The concept of free energy was developed by Hermann von Helmholtz, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |