|
FtsZ Filaments
FtsZ is a protein encoded by the ''ftsZ'' gene that assembles into a ring at the future site of bacterial cell division (also called the Z ring). FtsZ is a prokaryotic homologue of the eukaryotic protein tubulin. The initials FtsZ mean "Filamenting temperature-sensitive mutant Z." The hypothesis was that cell division mutants of ''E. coli'' would grow as filaments due to the inability of the daughter cells to separate from one another. FtsZ is found in almost all bacteria, many archaea, all chloroplasts and some mitochondria, where it is essential for cell division. FtsZ assembles the cytoskeletal scaffold of the Z ring that, along with additional proteins, constricts to divide the cell in two. History In the 1960s scientists screened for temperature sensitive mutations that blocked cell division at 42 °C. The mutant cells divided normally at 30°, but failed to divide at 42°. Continued growth without division produced long filamentous cells (Filamenting temperature se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in ''Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
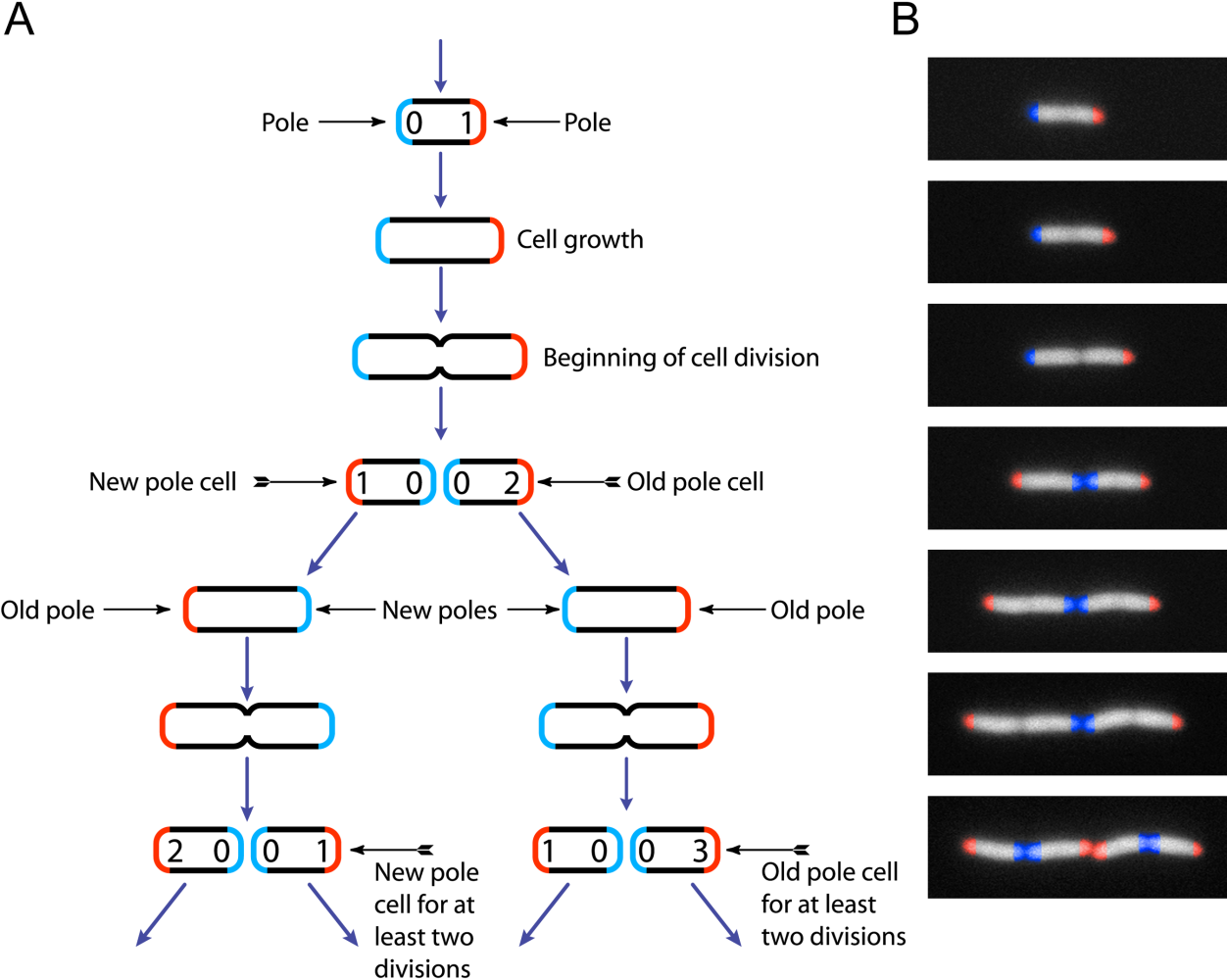
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FtsA
FtsA is a bacterial protein that is related to actin by overall structural similarity and in its ATP binding pocket. Along with other bacterial actin homologs such as MreB, ParM, and MamK, these proteins suggest that eukaryotic actin has a common ancestry. Like the other bacterial actins, FtsA binds ATP and can form actin-like filaments. The FtsA-FtsA interface has been defined by structural as well as genetic analysis. Although present in many diverse Gram-positive and Gram-negative species, FtsA is absent in actinobacteria and cyanobacteria. FtsA also is structurally similar to PilM, a type IV pilus ATPase. Function FtsA is required for proper cytokinesis in bacteria such as ''Escherichia coli'', ''Caulobacter crescentus'', and ''Bacillus subtilis''. Originally isolated in a screen for ''E. coli'' cells that could divide at 30˚C but not at 40˚C, FtsA stands for "filamentous temperature sensitive A". Many thermosensitive alleles of ''E. coli ftsA'' exist, and all map ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the generalized r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FtsZ Filaments
FtsZ is a protein encoded by the ''ftsZ'' gene that assembles into a ring at the future site of bacterial cell division (also called the Z ring). FtsZ is a prokaryotic homologue of the eukaryotic protein tubulin. The initials FtsZ mean "Filamenting temperature-sensitive mutant Z." The hypothesis was that cell division mutants of ''E. coli'' would grow as filaments due to the inability of the daughter cells to separate from one another. FtsZ is found in almost all bacteria, many archaea, all chloroplasts and some mitochondria, where it is essential for cell division. FtsZ assembles the cytoskeletal scaffold of the Z ring that, along with additional proteins, constricts to divide the cell in two. History In the 1960s scientists screened for temperature sensitive mutations that blocked cell division at 42 °C. The mutant cells divided normally at 30°, but failed to divide at 42°. Continued growth without division produced long filamentous cells (Filamenting temperature se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Segrosome
Segrosomes are protein complexes that ensure accurate segregation (partitioning) of plasmids or chromosomes during bacterial cell division. Just as higher forms of life have evolved a complex mitotic apparatus to partition duplicated DNA during cell division, bacteria require a specialized apparatus to partition their duplicated DNA. In bacteria, segrosomes perform the function similar to that performed by mitotic spindle. Therefore, segrosomes can be thought of as minimalist spindles. Segrosomes are usually composed of three basic components- the DNA (plasmid or chromosome) that needs to be segregated into daughter cells, a motor protein that provides the necessary physical forces for accomplishing the segregation and a DNA binding protein that connects the DNA and the motor protein, to form the complete segrosome complex. Motor proteins present in bacterial segrosomal complexes The majority of motor proteins participating in plasmid segrosomes are Walker-type or ParM type ATPas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Treadmilling
In molecular biology, treadmilling is a phenomenon observed within protein filaments of the cytoskeletons of many cells, especially in actin filaments and microtubules. It occurs when one end of a filament grows in length while the other end shrinks, resulting in a section of filament seemingly "moving" across a stratum or the cytosol. This is due to the constant removal of the protein subunits from these filaments at one end of the filament, while protein subunits are constantly added at the other end. Treadmilling was discovered by Wegner, who defined the thermodynamic and kinetic constraints. Wegner recognized that: “The equilibrium constant (K) for association of a monomer with a polymer is the same at both ends, since the addition of a monomer to each end leads to the same polymer.”; a simple reversible polymer can’t treadmill; ATP hydrolysis is required. GTP is hydrolyzed for microtubule treadmilling. Detailed process Dynamics of the filament The cytoskeleton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-form Bacteria
L-form bacteria, also known as L-phase bacteria, L-phase variants or cell wall-deficient (CWD) bacteria, are growth forms derived from different bacteria. They lack cell walls. Peptidoglycan ( murein) is absent. Two types of L-forms are distinguished: ''unstable L-forms'', spheroplasts that are capable of dividing, but can revert to the original morphology, and ''stable L-forms'', L-forms that are unable to revert to the original bacteria. Discovery and early studies L-form bacteria were first isolated in 1935 by Emmy Klieneberger-Nobel, who named them "L-forms" after the Lister Institute in London where she was working. She first interpreted these growth forms as symbionts related to pleuropneumonia-like organisms (PPLOs, later commonly called mycoplasmas). Mycoplasmas (now in scientific classification called '' Mollicutes''), parasitic or saprotrophic species of bacteria, also lack a cell wall (peptidoglycan/murein is absent).&nbsFull PDF/ref> Morphologically, they resemble L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |