|
Fluorobenzene
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds. Preparation PhF was first reported in 1886 by O. Wallach at the University of Bonn, who prepared the compound in two steps. Phenyldiazonium chloride was first converted to a triazene using piperidine: :[PhN2]Cl + 2 (CH2)5NH → PhN=N-N(CH2)5 + [(CH2)5NH2]Cl The triazine was then cleaved with hydrofluoric acid: :PhN=N-N(CH2)5 + 2 HF → PhF + N2 + [(CH2)5NH2]F Historical note: in Wallach's era, the element fluorine was symbolized with "Fl". Thus, his procedure is subtitled "Fluorbenzol, C6H5Fl". On the laboratory scale, PhF is prepared by the thermal decomposition of the benzenediazonium tetrafluoroborate: :PhN2BF4 → PhF + BF3 + N2 According to the procedure, solid [PhN2]BF4 is heated with a flame to initiate an exothermic reaction, which also affords bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Difluorobenzene
1,2-Difluorobenzene, also known as DFB, is an aromatic compound with formula CHF. This colorless flammable liquid is a solvent used in the electrochemical studies of transition metal complexes. Compared to most conventional halogenated aliphatic and aromatic solvents, it possesses an exceptionally high dielectric constant (ε0 = 13.8 at 300 K). Thus, it can be a suitable solvent for cationic, and/or highly electrophilic organometallic complexes. Synthesis Difluorobenzenes can be prepared by the Balz-Schiemann reaction, which entails conversion of diazonium tetrafluoroborate salts to their fluorides. The synthesis of 1,2-difluorobenzene starts with 2- fluoroaniline: : : The syntheses of 1,3- and 1,4-difluorobenzene proceed respectively from 1,3- and 1,4-diaminobenzene, which are doubly diazotized. Laboratory applications Organometallic derivatives of 1,2-difluorobenzene have been well developed. It is found to be a weaker base than benzene. 1,2-Difluorobenzene has been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorobenzenes
Fluorobenzenes are a group of aryl fluorides/halobenzenes consisting of one or more fluorine atoms as substituents on a benzene core. They have the formula C6H6–''n''F''n'', where ''n'' = 1–6 is the number of fluorine atoms. Depending on the number of fluorine substituents, there may be several constitutional isomers possible. * Monofluorobenzene * Difluorobenzene ** 1,2-Difluorobenzene ** 1,3-Difluorobenzene ** 1,4-Difluorobenzene * Trifluorobenzene ** 1,2,3-Trifluorobenzene ** 1,2,4-Trifluorobenzene ** 1,3,5-Trifluorobenzene * Tetrafluorobenzene ** 1,2,3,4-Tetrafluorobenzene ** 1,2,3,5-Tetrafluorobenzene ** 1,2,4,5-Tetrafluorobenzene * Pentafluorobenzene * Hexafluorobenzene See also *Chlorobenzenes *Bromobenzenes *Iodobenzenes Iodobenzenes are a group of aryl iodides/halobenzenes consisting of one or more iodine atoms as substituents on a benzene core. They have the formula C6H6–''n''I''n'', where ''n'' = 1–6 is the number of iodine atoms. Depending on the nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentafluoromethylbenzene
Pentafluoromethylbenzene is a synthetic organofluoride compound with the molecular formula . Synthesis The compound can be obtained by the Friedel–Crafts reaction of pentafluorobenzene with methyl chloride. Also, the compound can be prepared by reacting hexafluorobenzene with methyllithium. Physical properties Pentafluoromethylbenzene is a colorless, volatile liquid with a distinctive sweet smell. It is known for its high reactivity, low toxicity, and limited solubility. Additionally, it has the ability to form complexes with metals such as iron and copper. Chemical properties The presence of electron-withdrawing fluorine atoms on its aromatic ring significantly enhances the compound reactivity. Under the influence of light, pentafluoromethylbenzene reacts with bromine to form pentafluorobenzyl bromide. Uses This halogenated aromatic hydrocarbon is widely utilized in organic synthesis and analytical chemistry due to its unique characteristics. In scientific studies, the comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Fluoride
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bromine Br−1, or iodine I−). Aryl halides are distinct from haloalkanes (alkyl halides) due to significant differences in their methods of preparation, chemical reactivity, and physical properties. The most common and important members of this class are aryl chlorides, but the group encompasses a wide range of derivatives with diverse applications in organic synthesis, pharmaceuticals, and materials science. Classification according to halide Aryl fluorides Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals. The conversion of diazonium salts is a well established route to aryl fluorides. Thus, anilines are precursors to aryl fluorides. In the classic Schiemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change Δ''G''⚬ is negative." A strongly exothermic reaction will usually also be exergonic because Δ''H''⚬ makes a major contribution to Δ''G''⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Examples Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction: :4Fe + 3O2 → 2Fe2O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
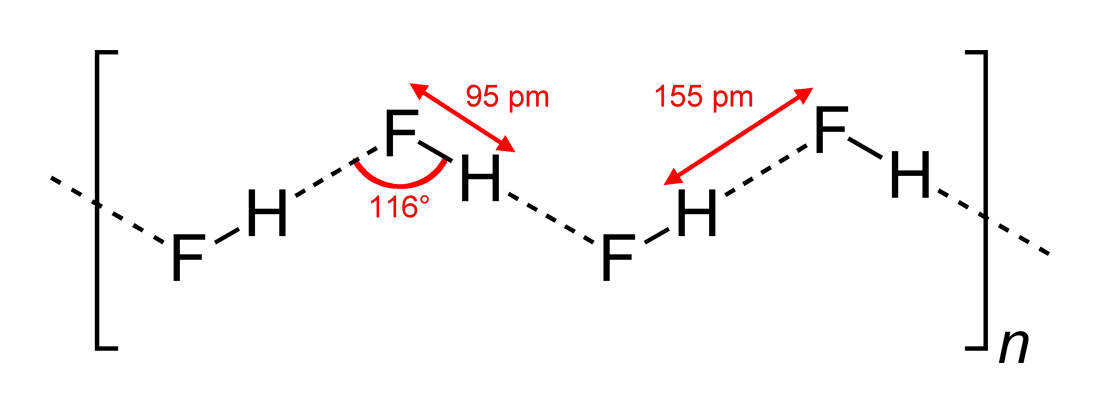
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance. Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-Dibromopropane, 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluorocarbene
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 ms, in solution and in the gas phase, respectively.Douglas A Jean Osteraas "Difluorocarbene Modification of Polymer and Fiber Surfaces," ''Journal of Applied Polymer''1969, volume 13, 1523-1535. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene, which is produced on an industrial scale as the precursor to Teflon (PTFE). Bonding in difluorocarbene In general, carbenes exist in either singlet or triplet states, which are often quite close in energy. Singlet carbenes have spin-paired electrons and a higher energy empty 2p orbital. In a triplet carbene, one electron occupies the hybrid orbital and the other is promoted to the 2p orbital.Jones. Maitland.''Organic Chemistry'', 3rd ed, W. W. Norton, 2005, 460-465. . For most carbenes, the triplet state is more stable than the corresponding singlet. In the case of fluorinated carbenes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimer (chemistry), dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction. This dimer can be retro-Diels–Alder reaction, restored by heating to give the monomer. The compound is mainly used for the production of cyclopentene and its derivatives. It is popularly used as a precursor to the cyclopentadienyl anion (Cp−), an important ligand in cyclopentadienyl complexes in organometallic chemistry. Production and reactions Cyclopentadiene production is usually not distinguished from dicyclopentadiene since they interconvert. They are obtained from coal tar (about 10–20 g/tonne, t) and by steam Cracking (chemistry), cracking of Petroleum naphtha, naphtha (about 14 kg/t) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |