|
Fluorine-20
Fluorine (9F) has 18 known isotopes ranging from to (with the exception of ) and two isomers ( and ). Only fluorine-19 is stable and naturally occurring in more than trace quantities; therefore, fluorine is a monoisotopic and mononuclidic element. The longest-lived radioisotope is ; it has a half-life of . All other fluorine isotopes have half-lives of less than a minute, and most of those less than a second. The least stable known isotope is , whose half-life is , corresponding to a resonance width of . List of isotopes , - , , style="text-align:right" , 9 , style="text-align:right" , 4 , # , , p ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , 1/2+# , , , - , , style="text-align:right" , 9 , style="text-align:right" , 5 , , [] , p ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , 2− , , , - , , style ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiopharmacology
Radiopharmacology is radiochemistry applied to medicine and thus the pharmacology of radiopharmaceuticals ( medicinal radiocompounds, that is, pharmaceutical drugs that are radioactive). Radiopharmaceuticals are used in the field of nuclear medicine as radioactive tracers in medical imaging and in therapy for many diseases (for example, brachytherapy). Many radiopharmaceuticals use technetium-99m (Tc-99m) which has many useful properties as a gamma-emitting tracer nuclide. In the book ''Technetium'' a total of 31 different radiopharmaceuticals based on Tc-99m are listed for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood and tumors. The term ''radioisotope'', which in its general word sense, sense refers to any radioactive isotope (radionuclide), has historically been used to refer to all radiopharmaceuticals, and this usage remains common. Technically, however, many radiopharmaceuticals incorporate a radioa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mean Lifetime
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate called the exponential decay constant, disintegration constant, rate constant, or transformation constant: :\frac = -\lambda N. The solution to this equation (see derivation below) is: :N(t) = N_0 e^, where is the quantity at time , is the initial quantity, that is, the quantity at time . Measuring rates of decay Mean lifetime If the decaying quantity, ''N''(''t''), is the number of discrete elements in a certain set, it is possible to compute the average length of time that an element remains in the set. This is called the mean lifetime (or simply the lifetime), where the exponential time constant, \tau, relates to the decay rate constant, λ, in the following way: :\tau = \frac. The mean lifetime can be looked at as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Activity
Specific activity is the activity per unit mass of a radionuclide and is a physical property of that radionuclide. Activity is a quantity (for which the SI unit is the becquerel) related to radioactivity, and is defined as the number of radioactive transformations per second that occur in a particular radionuclide. The unit of activity is the becquerel (Bq), which is defined as one radioactive decay per second. The older, non-SI unit of activity is the curie (Ci), which is radioactive decay per second. Another unit of activity is the Rutherford, which is defined as radioactive decay per second. Since the probability of radioactive decay for a given radionuclide within a set time interval is fixed (with some slight exceptions, see changing decay rates), the number of decays that occur in a given time of a given mass (and hence a specific number of atoms) of that radionuclide is also a fixed (ignoring statistical fluctuations). Thus, specific activity is defined as the act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine-19 NMR
Fluorine-19 nuclear magnetic resonance spectroscopy (fluorine NMR or 19F NMR) is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy. Operational details 19F has a nuclear spin (I) of and a high gyromagnetic ratio. Consequently, this isotope is highly responsive to NMR measurements. Furthermore, 19F comprises 100% of naturally occurring fluorine. The only other highly sensitive spin NMR-active nuclei that are monoisotopic (or nearly so) are 1H and 31P. Indeed, the 19F nucleus is the third most receptive NMR nucleus, after the 3H nucleus and 1H nucleus. The 19F NMR chemical shifts span a range of ''ca.'' 800 ppm. For ''organo''fluorine compounds the range is narrower, being ''ca.'' -50 to -70 ppm (for CF3 groups) to -200 to -220 ppm (for CH2F groups). T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundance of such stable isotopes can be measured experimentally (isotope analysis), yielding an isotope ratio that can be used as a research tool. Theoretically, such stable isotopes could include the radiogenic daughter products of radioactive decay, used in radiometric dating. However, the expression stable-isotope ratio is preferably used to refer to isotopes whose relative abundances are affected by isotope fractionation in nature. This field is termed stable isotope geochemistry. Stable-isotope ratios Measurement of the ratios of naturally occurring stable isotopes (isotope analysis) plays an important role in isotope geochemistry, but stable isotopes (mostly hydrogen, carbon, nitrogen, oxygen and sulfur) are also finding uses in ecologic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
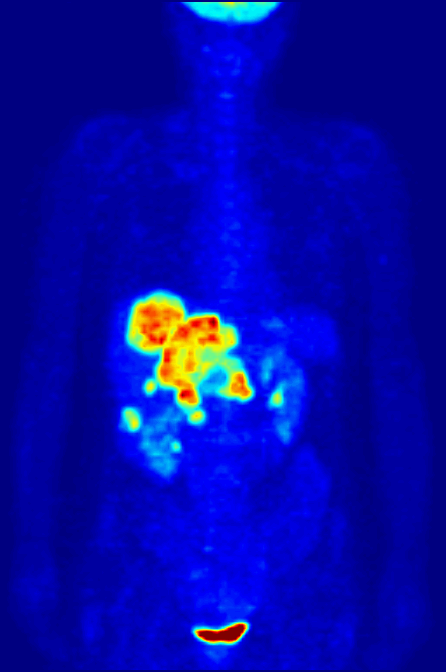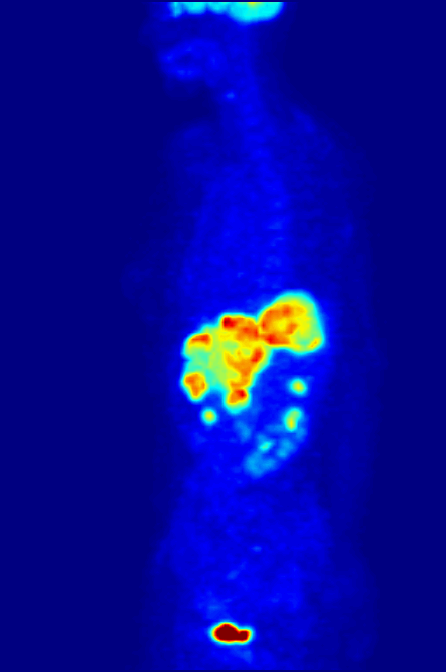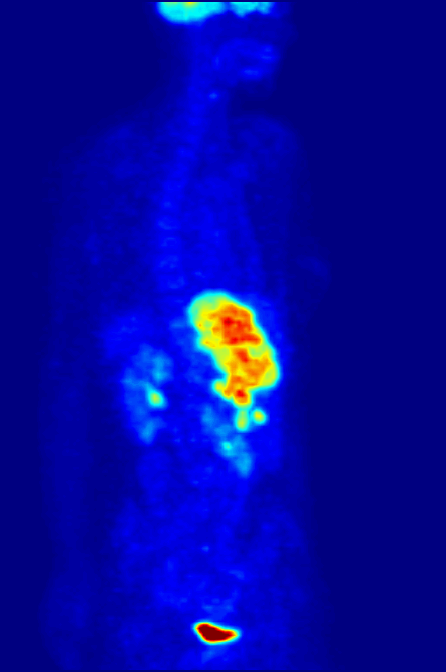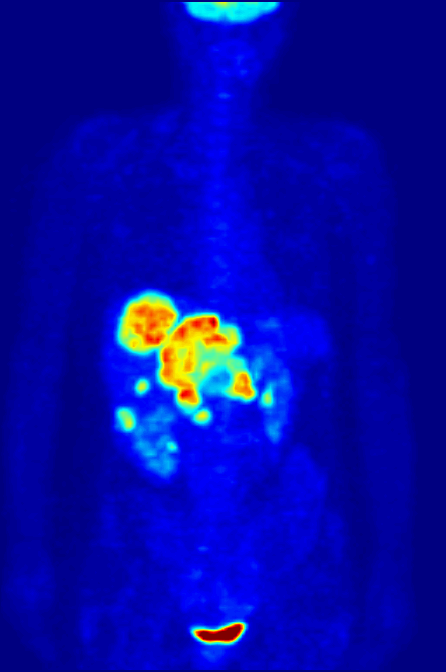
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example: * Fluorodeoxyglucose ( 18F">sup>18FDG or FDG) is commonly used to detect cancer; * 18Fodium fluoride">sup>18Fodium fluoride (Na18F) is widely used for detecting bone formation; * Oxygen-15 (15O) is sometimes used to measure blood flow. PET is a common imaging technique, a medical scintillography technique used in nuclear medicine. A radiopharmaceutical – a radioisotope attached to a drug – is injected into the body as a radioactive tracer, tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is emitted, and when the positron interacts with an or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |