 |
Flory–Huggins Solution Theory
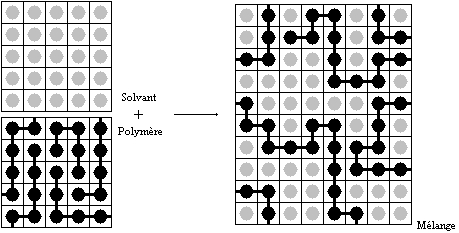
Flory–Huggins solution theory is a lattice model (physics), lattice model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecule, molecular sizes in adapting the usual expression (mathematics), expression for the entropy of mixing. The result is an equation for the Gibbs free energy change \Delta G_ for mixing a polymer with a solvent. Although it makes simplifying assumptions, it generates useful results for interpreting experiments. Theory The thermodynamic potentials, thermodynamic equation for the Gibbs energy change accompanying mixing at constant temperature and (external) pressure is : \Delta G_ = \Delta H_ - T\Delta S_ A change, denoted by \Delta, is the number, value of a Variable (mathematics), variable for a Solution (chemistry), solution or mixture minus the values for the pure Component (thermodynamics), components considered separately. The objective is to find explicit formulas for \Delta H_ and \Delta S_, the en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |