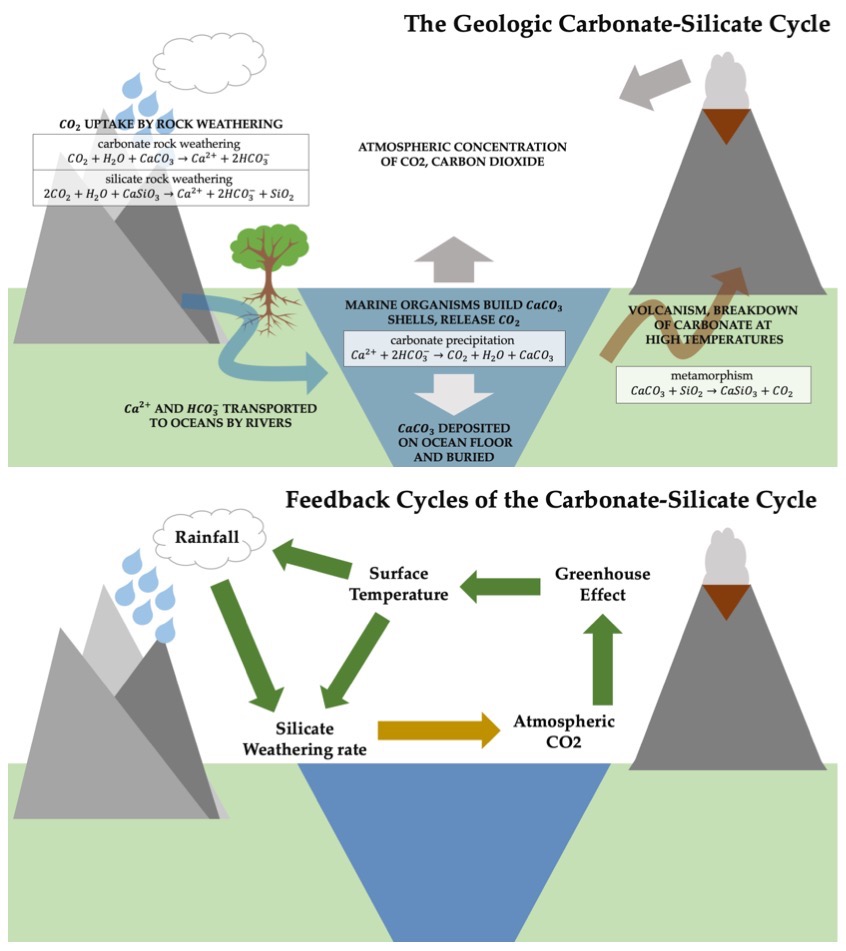
|
Enhanced Weathering
Enhanced weathering, also termed ocean alkalinity enhancement when proposed for carbon credit systems, is a process that aims to accelerate the natural weathering by spreading finely ground silicate rock, such as basalt, onto surfaces which speeds up chemical reactions between rocks, water, and air. It also removes carbon dioxide () from the atmosphere, permanently storing it in solid carbonate minerals or ocean alkalinity. The latter also slows ocean acidification. Enhanced weathering is a chemical approach to remove carbon dioxide involving land-based or ocean-based techniques. One example of a land-based enhanced weathering technique is in-situ carbonation of silicates. Ultramafic rock, for example, has the potential to store hundreds to thousands of years' worth of CO2 emissions, according to estimates. Ocean-based techniques involve alkalinity enhancement, such as grinding, dispersing, and dissolving olivine, limestone, silicates, or calcium hydroxide to address ocean acidif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Credit
Carbon offsetting is a carbon trading mechanism that enables entities to compensate for offset greenhouse gas emissions by investing in projects that reduce, avoid, or remove emissions elsewhere. When an entity invests in a carbon offsetting program, it receives carbon credit or offset credit, which account for the net climate benefits that one entity brings to another. After certification by a government or independent certification body, credits can be traded between entities. One carbon credit represents a reduction, avoidance or removal of one metric tonne of carbon dioxide or its Global warming potential, carbon dioxide-equivalent (CO2e). A variety of greenhouse gas reduction projects can qualify for offsets and credits depending on the scheme. Some include forestry projects that avoid logging and plant saplings, renewable energy projects such as wind farms, biomass energy, biogas digesters, Hydroelectric Dams, hydroelectric dams, as well as Efficient energy use, energy ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The Geology, geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock (geology), rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate (geology), aggregate of two or more different types of minerals, spaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the specific material under consideration. Solids also always possess the least amount of kinetic energy per atom/molecule relative to other phases or, equivalently stated, solids are formed when matter in the liquid / gas phase is cooled below a certain temperature. This temperature is called the melting point of that substance and is an intrinsic property, i.e. independent of how much of the matter there is. All matter in solids can be arranged on a microscopic scale under certain conditions. Solids are characterized by structural rigidity and resistance to applied external forces and pressure. Unlike liquids, solids do not flow to take on the shape of their container, nor do they expand to fill the entire available volume like a gas. Much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Forsterite
Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich Endmember, end-member of the olivine solid solution series. It is Isomorphism (crystallography), isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group ''Pbnm'') with cell parameters ''a'' 4.75 Ångström, Å (0.475 Nanometre, nm), ''b'' 10.20 Å (1.020 nm) and ''c'' 5.98 Å (0.598 nm). Forsterite is associated with Igneous rock, igneous and metamorphic rocks and has also been found in meteorites. In 2005 it was also found in cometary dust returned by the Stardust probe. In 2011 it was observed as tiny crystals in the dusty clouds of gas around a forming star. Two Polymorphism (materials science), polymorphs of forsterite are known: wadsleyite (also orthorhombic) and ringwoodite (isometric, cubic crystal system). Both are mainly known from meteorites. Peridot is the gemstone variety of forsterite olivine. Comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate Mineral
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, the crystalline forms of silica (silicon dioxide, ) are usually considered to be Silicate mineral#Tectosilicates, tectosilicates, and they are classified as such in the Dana system (75.1). However, the Nickel-Strunz system classifies them as oxide minerals (4.DA). Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal **Calcite CaCO3 **Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 **Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 **Cerussite PbCO3 **Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 **Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal **Ankerite CaFe(CO3)2 **Dolomite (mineral), Dolomite CaMg(CO3)2 **Huntite Mg3Ca(CO3)4 **Minrecordite CaZn(CO3)2 **Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 **Malachite Cu2CO3(OH)2 **Rosasite (Cu,Zn)2CO3(OH)2 **Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikaite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids. In inorganic chemistry and geology, carbonation is common. Metal hydroxides (MOH) and metal oxides (M'O) react with CO2 to give bicarbonates and carbonates: :MOH + CO2 → M(HCO3) :M'O + CO2 → M'CO3 Selected carbonations Carbonic anhydrase In mammalian physiology, transport of carbon dioxide to the lungs involves a carbonation reaction catalyzed by the enzyme carbonic anhydrase. In the absence of such catalysts, carbon dioxide cannot be expelled sufficient rate to support metabolic needs. The enzyme harbors a zinc aquo complex, which captures carbon dioxide to give a zinc bicarbonate: : Behavior of concrete In reinforced concrete, the chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |