|
Deiodinase
Deiodinase (or "Monodeiodinase") is a peroxidase enzyme that is involved in the activation or deactivation of thyroid hormones. Types Types of deiodinases include: Iodothyronine deiodinases catalyze release of iodine directly from the thyronine hormones. They are selenocysteine-dependent membrane proteins with a catalytic domain resembling Peroxiredoxins (Prx). Three related isoforms, deiodinase type I, II, and III, contribute to activation and inactivation of the initially released hormone precursor T4 (thyroxine) into T3 (triiodothyronine) or rT3 ( reverse triiodothyronine) in target cells. The enzymes catalyze a reductive elimination of iodine (the different isoforms attack different thyronine positions), thereby oxidizing themselves similar to Prx, followed by a reductive recycling of the enzyme. Iodotyrosine deiodinase contributes to breakdown of thyroid hormones. It releases iodine, for renewed use, from iodinated tyrosines resulting from catabolism of iodothyronines. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodothyronine Deiodinase
Iodothyronine deiodinases ( and ) are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine (T4), the precursor of 3,5,3'-triiodothyronine (T3) is transformed into T3 by deiodinase activity. T3, through binding a nuclear thyroid hormone receptor, influences the expression of genes in practically every vertebrate cell. Iodothyronine deiodinases are unusual in that these enzymes contain selenium, in the form of an otherwise rare amino acid selenocysteine. These enzymes are not to be confused with the iodotyrosine deiodinases that are also deiodinases, but not members of the iodothyronine family. The iodotyrosine deiodinases (unlike the iodothyronine deiodinases) do ''not'' use selenocysteine or selenium. The iodotyrosine enzymes work on iodinated single tyrosine residue molecules to scavenge iodine, and do not use as substrates the double-tyrosine residue molecules of the various iodo thyronines. Activation and inactivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DIO1
Iodothyronine deiodinases ( and ) are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine (T4), the precursor of 3,5,3'-triiodothyronine (T3) is transformed into T3 by deiodinase activity. T3, through binding a nuclear thyroid hormone receptor, influences the expression of genes in practically every vertebrate cell. Iodothyronine deiodinases are unusual in that these enzymes contain selenium, in the form of an otherwise rare amino acid selenocysteine. These enzymes are not to be confused with the iodotyrosine deiodinases that are also deiodinases, but not members of the iodothyronine family. The iodotyrosine deiodinases (unlike the iodothyronine deiodinases) do ''not'' use selenocysteine or selenium. The iodotyrosine enzymes work on iodinated single tyrosine residue molecules to scavenge iodine, and do not use as substrates the double-tyrosine residue molecules of the various iodo thyronines. Activation and inactivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DIO3
Thyroxine 5-deiodinase also known as type III iodothyronine deiodinase (EC number 1.21.99.3) is an enzyme that in humans is encoded by the ''DIO3'' gene. This enzyme catalyses the following chemical reaction : 3,3',5'-triiodo-L-thyronine + iodide + A + H+ \rightleftharpoons L-thyroxine + AH2 The protein encoded by this intronless gene belongs to the iodothyronine deiodinase family. It catalyzes the inactivation of thyroid hormone by inner ring deiodination of the prohormone thyroxine (T4) and the bioactive hormone 3,3',5-triiodothyronine (T3) to inactive metabolites, 3,3',5'-triiodothyronine (RT3) and 3,3'-diiodothyronine (T2), respectively. This enzyme is highly expressed in the pregnant uterus, placenta, fetal and neonatal tissues, suggesting that it plays an essential role in the regulation of thyroid hormone inactivation during embryological development. Discovery The gene was mapped to chromosome 14q32 using fluorescence in situ hybridization (FISH) in 1998. Struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DIO2
Type II iodothyronine deiodinase (''iodothyronine 5'-deiodinase'', ''iodothyronine 5'-monodeiodinase'') is an enzyme that in humans is encoded by the ''DIO2'' gene. Function The protein encoded by this gene belongs to the iodothyronine deiodinase family. It activates thyroid hormone by converting the prohormone thyroxine (T4) by outer ring deiodination (ORD) to bioactive 3,3',5-triiodothyronine (T3). It is highly expressed in the thyroid, and may contribute significantly to the relative increase in thyroidal T3 production in patients with Graves' disease and thyroid adenomas. This protein contains selenocysteine (Sec) residues encoded by the UGA codon, which normally signals translation termination. The 3' UTR of Sec-containing genes have a common stem-loop structure, the Sec insertion sequence (SECIS), which is necessary for the recognition of UGA as a Sec codon rather than as a stop signal. Alternative splicing results in multiple transcript variants encoding different isof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodotyrosine Deiodinase
Iodotyrosine deiodinase, also known as iodotyrosine dehalogenase 1, is a type of deiodinase enzyme that scavenges iodide by removing it from iodinated tyrosine residues in the thyroid gland. These iodinated tyrosines are produced during thyroid hormone biosynthesis. The iodide that is scavenged by iodotyrosine deiodinase is necessary to again synthesize the thyroid hormones. After synthesis, the thyroid hormones circulate through the body to regulate metabolic rate, protein expression, and body temperature. Iodotyrosine deiodinase is thus necessary to keep levels of both iodide and thyroid hormones in balance. Dehalogenation in aerobic organisms is usually done through oxidation and hydrolysis; however, iodotyrosine deiodinase uses reductive dehalogenation. Iodotyrosine deiodinase and iodothyronine deiodinase have been determined as the only two known enzymes to catalyze reductive dehalogenation in mammals. Although these two enzymes perform similar functions, they are structural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides. Functionality Peroxidases typically catalyze a reaction of the form: :ROOR' + \overset + 2H+ -> ce + R'OH Optimal substrates For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides. Peroxidases can contain a heme cofactor in their active sites, or alternately redox-active cysteine or selenocysteine residues. The nature of the electron donor is very dependent on the structure of the enzyme. * For example, horseradish peroxidase can use a variety of organic compounds as electron donors and acceptors. Horseradish peroxidase has an accessible active site, and many compounds can reach the site of the reaction. * On the other hand, for an enzyme such as cytochrome c peroxidase, the com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reverse Triiodothyronine
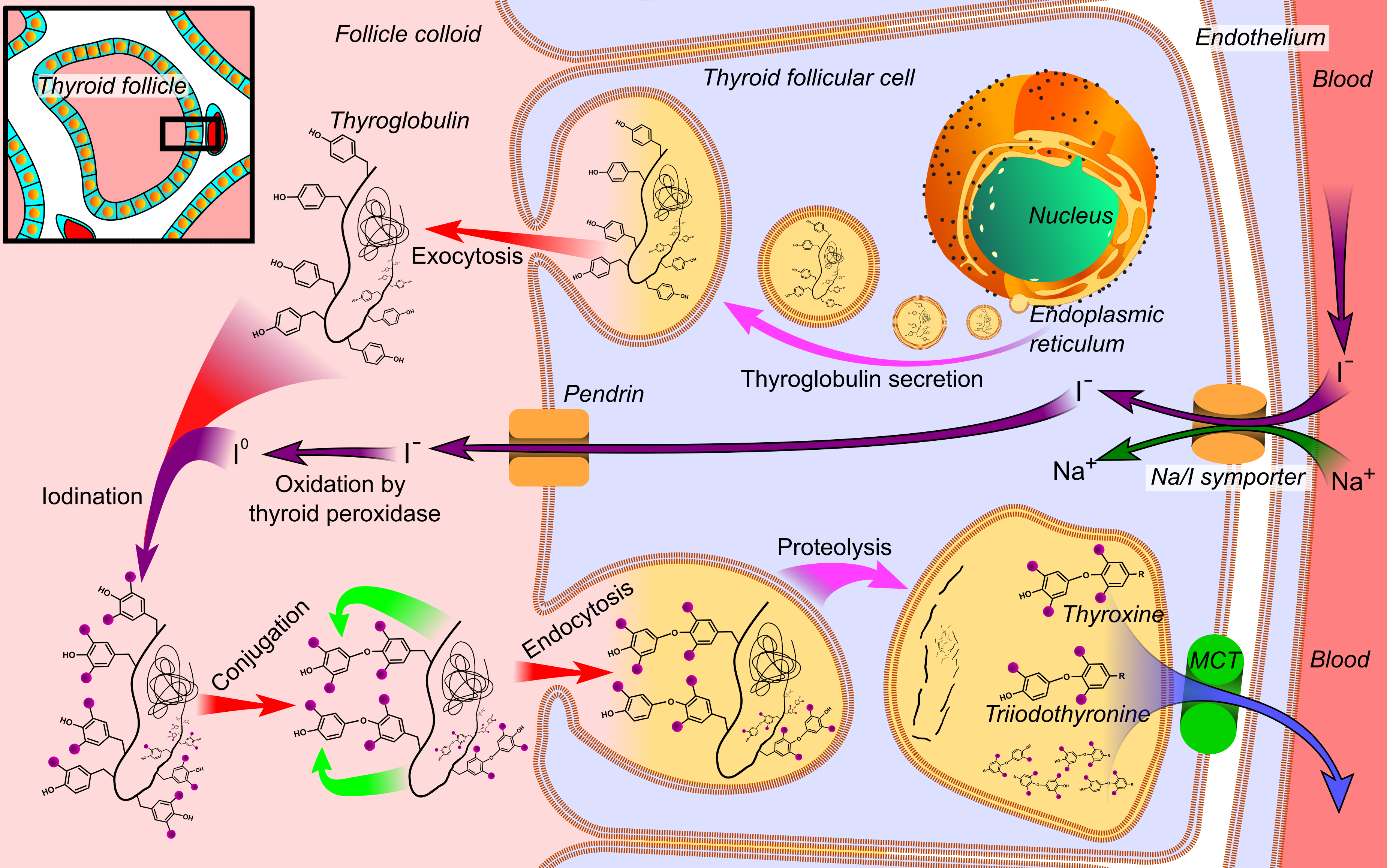
Reverse triiodothyronine (3,3′,5′-triiodothyronine, reverse T3, or rT3) is an isomer of triiodothyronine (3,5,3′ triiodothyronine, T3). Reverse T3 is the third-most common iodothyronine the thyroid gland releases into the bloodstream, at 0.9%; tetraiodothyronine (levothyroxine, T4) constitutes 90% and T3 is 9%. However, 95% of rT3 in human blood is made elsewhere in the body, as enzymes remove a particular iodine atom from T4. The production of hormone by the thyroid gland is controlled by the hypothalamus and pituitary gland. The physiological activity of thyroid hormone is regulated by a system of enzymes that activate, inactivate or simply discard the prohormone T4 and in turn functionally modify T3 and rT3. These enzymes operate under complex direction of systems including neurotransmitters, hormones, markers of metabolism and immunological signals. The levels of rT3 increase in conditions such as euthyroid sick syndrome Euthyroid sick syndrome (ESS) is a state of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium Deficiency
Selenium deficiency occurs when an organism lacks the required levels of selenium, a critical nutrient in many species. Deficiency, although relatively rare in healthy well-nourished individuals, can have significant negative results, affecting the health of the heart and the nervous system; contributing to depression, anxiety, and dementia; and interfering with reproduction and gestation. Signs and symptoms Selenium deficiency in combination with Coxsackievirus infection can lead to Keshan disease, which is potentially fatal. Selenium deficiency also contributes (along with iodine deficiency) to Kashin-Beck disease. The primary symptom of Keshan disease is myocardial necrosis, leading to weakening of the heart. Kashin-Beck disease results in atrophy, degeneration and necrosis of cartilage tissue. Keshan disease also makes the body more susceptible to illness caused by other nutritional, biochemical, or infectious diseases. Selenium is also necessary for the conversion of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Mononucleotide
Flavin mononucleotide (FMN), or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as the prosthetic group of various oxidoreductases, including NADH dehydrogenase, as well as cofactor in biological blue-light photo receptors. During the catalytic cycle, a reversible interconversion of the oxidized (FMN), semiquinone (FMNH•), and reduced (FMNH2) forms occurs in the various oxidoreductases. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. In its role as blue-light photo receptor, (oxidized) FMN stands out from the 'conventional' photo receptors as the signaling state and not an E/Z isomerization. It is the principal form in which riboflavin is found in cells and tissues. It requires more energy to produce, but is more soluble than riboflavin. In cells, FMN occurs freely circulating but also in several covalently bou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triiodothyronine
Triiodothyronine, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate. Production of T3 and its prohormone thyroxine (T4) is activated by thyroid-stimulating hormone (TSH), which is released from the anterior pituitary gland. This pathway is part of a closed-loop feedback process: Elevated concentrations of T3, and T4 in the blood plasma inhibit the production of TSH in the anterior pituitary gland. As concentrations of these hormones decrease, the anterior pituitary gland increases production of TSH, and by these processes, a feedback control system stabilizes the level of thyroid hormones in the bloodstream. T3 is the true hormone. Its effects on target tissues are roughly four times more potent than those of T4. Of the thyroid hormone that is produced, just about 20% is T3, whereas 80% is produced as T4. Roughly 85% of the circulating T3 is la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |