|
Diterpene
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important Chemical compound, compounds such as retinol, retinal, and phytol. Some diterpenes are known to be antimicrobial and anti-inflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one Isopentenyl pyrophosphate, IPP unit to Farnesyl pyrophosphate, FPP to form geranylgeranyl pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and Cytochrome P450, cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Terpenes
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control. Terpenes are classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine. The one terpene that has major applications is natural rubber (i.e., polyisoprene). The possibility that other terpenes could be used as precursors to produce synthetic polymers has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Labdane
Labdane is a natural bicyclic diterpene. It forms the structural core for a wide variety of natural products collectively known as ''labdanes'' or ''labdane diterpenes''. The labdanes were so named because the first members of the class were originally obtained from labdanum, a resin derived from the gum rockrose. A variety of biological activities have been determined for labdane diterpenes including antibacterial, antifungal, antiprotozoal, and anti-inflammatory activities. Natural labdanes in tree resin are believed to be the precursors of amber, which polymerise under great pressure. Example labdane derivatives * Forskolin * Galanolactone * Isocupressic acid - is an abortifacient component of ''Cupressus macrocarpa''. * Medigenin *Sclareol Sclareol is a fragrant chemical compound found in '' Salvia sclarea'', from which it derives its name. It is classified as a bicyclic diterpene alcohol. It is an amber colored solid with a sweet, balsamic scent. In an experime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Geranylgeranyl Pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, which is its primary use in human cells. It is formed from farnesyl pyrophosphate by the addition of an isoprene unit from isopentenyl pyrophosphate. In ''Drosophila'', geranylgeranyl pyrophosphate is synthesised by HMG-CoA encoded by the Columbus gene. Geranylgeranyl pyrophosphate is utilised as a chemoattractant for migrating germ cells that have traversed the midgut epithelia. The attractant signal is produced at the gonadal precursors, directing the germ cells to these sites, where they will differentiate into eggs and spermatozoa (sperm). Related compounds * Farnesyl pyrophosphate * Geranylgeraniol Geranylgeraniol is a diterpenoid alcohol. It is a colorless waxy solid. It is an important intermediate in the biosynthesis of other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Stemodene
Stemodene is a labdane-related diterpene whose corresponding class I terpene synthase has been discovered in rice and subsequently cloned and functionally characterized. The gene responsible for stemodene production has not been found in the completed rice genome, thus suggesting that perhaps other genes are as yet undiscovered in the "completed" genome. Stemarene synthase demonstrates high sequence homology with stemodene synthase, thus accounting for the latter's discovery by Dana Morrone in 2005. Additionally, the corresponding olefin In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins. The International Union of Pu ... produced by each cyclase shows structural similarities and is derived from the common precursor of ''syn''-copalyl diphosphate. References Diterpenes Cyclopentanes {{alkanederivativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cembrene A
Cembrene A, or sometimes neocembrene, is a natural monocyclic diterpene isolated from corals of the genus ''Nephthea''. It is a colorless oil with a faint wax-like odor. Cembrene A is a trail pheromone for termites; however, the chemical structure of cembrene is central to a very wide variety of other natural products found both in plants and in animals.''Terpenes: Flavors, Fragrances, Pharmaca, Pheromones'', Eberhard Breitmaier, page 7. ''Pinus leucodermis'' tree bark and wood essential oils contain a high percentage of cembrene. Cembrenes are biosynthesized by macrocyclization of geranylgeranyl pyrophosphate Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, whic .... References {{reflist Diterpenes Alkene derivatives Insect ecology Insect pheromones Cycloalkenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
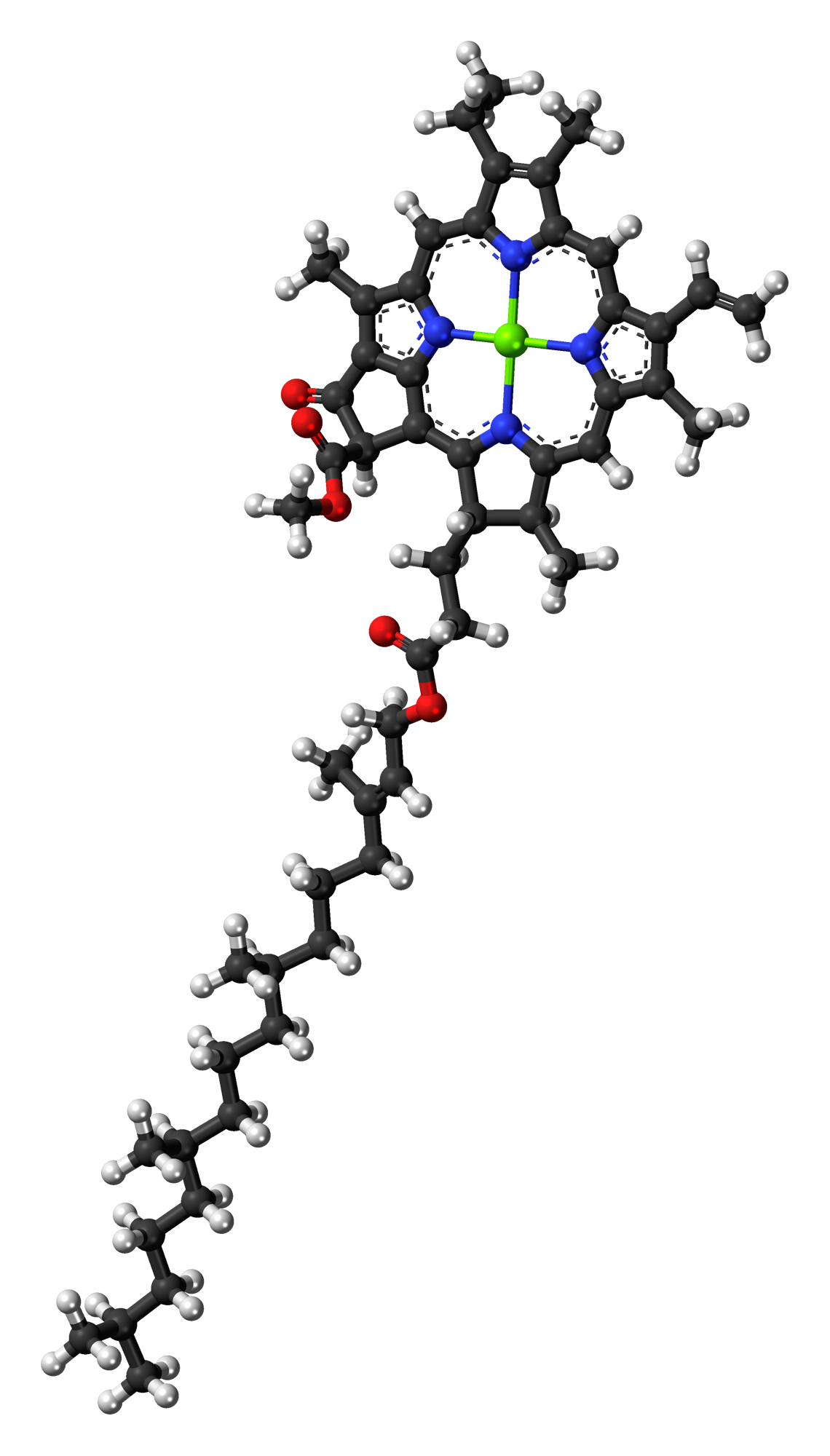
Chlorophyll A
} Chlorophyll ''a'' is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll ''a'' also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located. Distribution of chlorophyll ''a'' Chlorophyll ''a'' is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chloroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Farnesyl Pyrophosphate
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is the precursor to all sesquiterpenes, which comprises thousands of compounds. These include all sesquiterpenes as well as sterols and carotenoids. It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for ''N''-glycosylation). Biosynthesis Farnesyl pyrophosphate synthase (a prenyl transferase) catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate: : Pharmacology The above reactions are inhibited by bisphosphonates (used for osteoporosis). Farnesyl pyrophosphate is a selective agonist of TRPV3. Related compounds * Farnesene * Farnesol * Geranyl pyrophosphate *Geranylgeranyl pyrophosphate Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Stemarene
Stemarene is a diterpene hydrocarbon can be produced biosynthetically through enzyme An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ... extracts from rice. References Diterpenes Cyclopentanes {{alkanederivative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sclarene
Sclarene is a diterpene present in the foliage of '' Podocarpus hallii''. References Diterpenes Decalins Polyenes Vinylidene compounds {{organic-compound-stub ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phytol
Phytol (florasol, phytosol) is an acyclic hydrogenated diterpene alcohol that is used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1, as well as in the fragrance industry. Its other commercial uses include cosmetics, shampoos, toilet soaps, and detergents, as well as in some cannabis distillates as a diluent or for flavoring. It smells grassy and dominates the aroma of certain green teas. Its worldwide use has been estimated to be approximately 0.1–1.0 metric tons per year. Pharmacology Humans Refsum disease (also known as adult Refsum disease) is an autosomal recessive disorder that results in the accumulation of toxic stores of phytanic acid in tissues and frequently manifests as a variable combination of peripheral polyneuropathy, cerebellar ataxia, retinitis pigmentosa, anosmia, and hearing loss. Although humans cannot derive phytanic acid from chlorophyll, they can convert free phytol into phytanic acid. Thus, patients with Refsum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Taxadiene
Taxadiene (taxa-4,11-diene) is a diterpene. Taxadiene is the first committed intermediate in the synthesis of taxol. Six hydroxylation reactions, and a few others, are needed to convert taxadiene to baccatin III. Enzymatically, taxadiene is produced from geranylgeranyl pyrophosphate by taxadiene synthase. A biochemical gram-scale production of taxadiene has been reported in 2010 using genetically engineered ''Escherichia coli ''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly fo ...''. References Dienes Taxanes Diterpenes {{Ether-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |