|
Costamere
The costamere is a structural-functional component of striated muscle cells which connects the sarcomere of the muscle to the cell membrane (i.e. the sarcolemma).20: 2327-2331 Costameres are sub-sarcolemmal protein assemblies circumferentially aligned in register with the Z-disk of peripheral myofibrils. They physically couple force-generating sarcomeres with the sarcolemma in striated muscle cells and are thus considered one of several "Achilles' heels" of skeletal muscle, a critical component of striated muscle morphology which, when compromised, is thought to directly contribute to the development of several distinct myopathies. The dystrophin-associated protein complex, also referred to as the dystrophin-associated glycoprotein complex (DGC or DAGC), contains various integral and peripheral membrane proteins such as dystroglycans and sarcoglycans, which are thought to be responsible for linking the internal cytoskeletal system of individual myofibers to structural proteins wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystrophin
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa Dystrophin is coded for by the ''DMD'' gene – the largest known human gene, covering 2.4 megabases (0.08% of the human genome) at locus Xp21. The primary transcript in muscle measures about 2,100 kilobases and takes 16 hours to transcribe; the mature mRNA measures 14.0 kilobases. The 79-exon muscle transcript codes for a protein of 3685 amino acid residues. Spontaneous or inherited mutations in the dystrophin gene can cause different forms of muscular dystrophy, a disease characteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystrophin-associated Protein Complex
The dystrophin-associated protein complex, also known as the dystrophin-associated glycoprotein complex is a multiprotein complex that includes dystrophin and the dystrophin-associated proteins. It is one of the two protein complexes that make up the costamere in striated muscle cells. The other complex is the ''integrin-vinculin-talin complex''. Structure The dystrophin-associated protein complex includes dystrophin. Dystrophin binds to actin of the cytoskeleton, and also to proteins in the extracellular matrix. The dystrophin-associated protein complex also contains dystrophin-associated proteins. This includes a four subunit sarcoglycan complex, which is fixed to dystrophin in muscle cells. In the epithelia of the kidney, dystrophin may be replaced with utrophin. Aquaporin 4 may be connected to the dystrophin-associated protein complex. Function The dystrophin-associated protein complex is important for cell structure and cell signalling. It is one of two protein c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystrophin-associated Glycoprotein Complex
The dystrophin-associated protein complex, also known as the dystrophin-associated glycoprotein complex is a multiprotein complex that includes dystrophin and the dystrophin-associated proteins. It is one of the two protein complexes that make up the costamere in striated muscle cells. The other complex is the ''integrin-vinculin-talin complex''. Structure The dystrophin-associated protein complex includes dystrophin. Dystrophin binds to actin of the cytoskeleton, and also to proteins in the extracellular matrix. The dystrophin-associated protein complex also contains dystrophin-associated proteins. This includes a four subunit sarcoglycan complex, which is fixed to dystrophin in muscle cells. In the epithelia of the kidney, dystrophin may be replaced with utrophin. Aquaporin 4 may be connected to the dystrophin-associated protein complex. Function The dystrophin-associated protein complex is important for cell structure and cell signalling. It is one of two protein comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
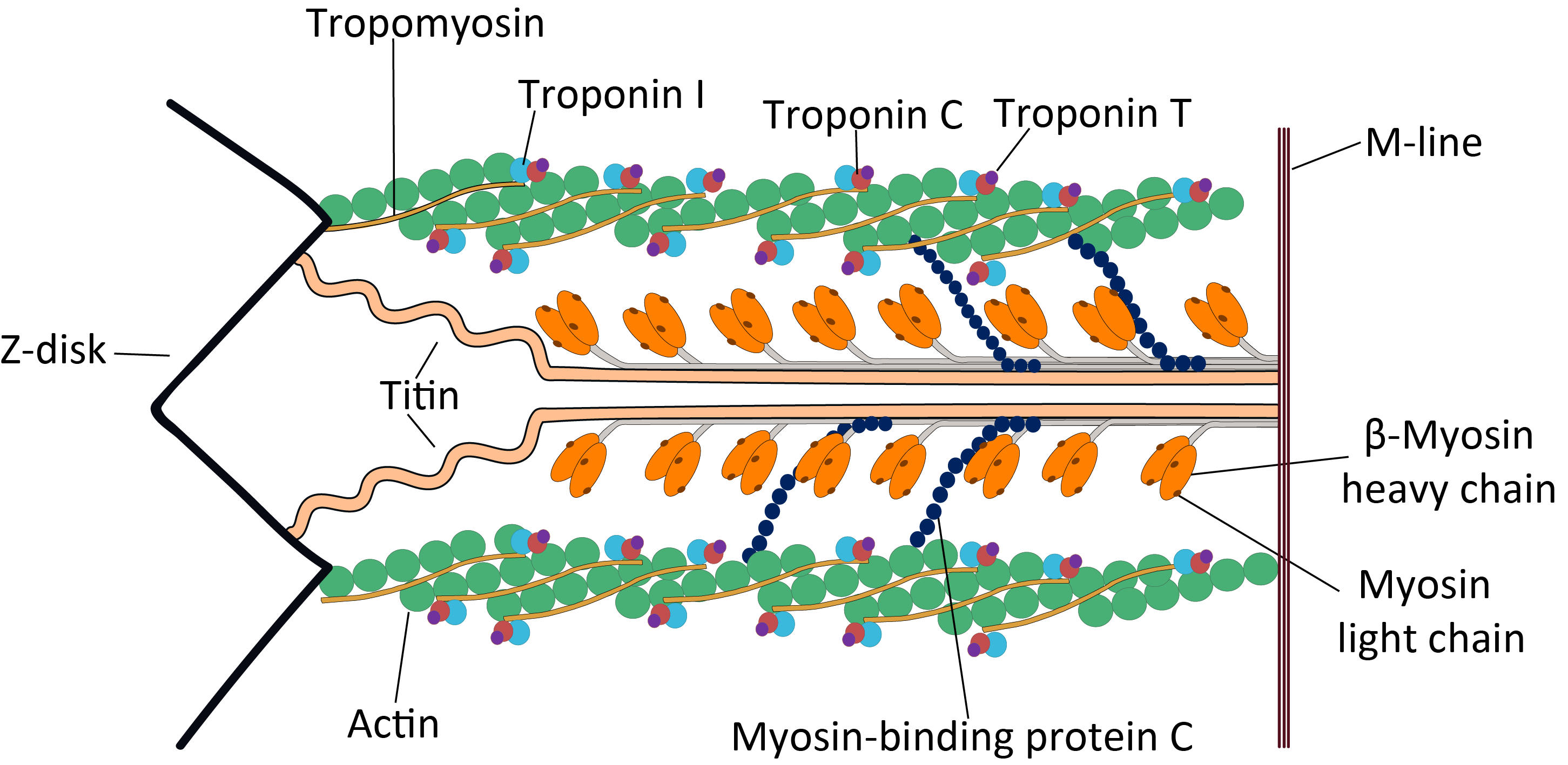
Sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called muscle fibers or myofibers) which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long, fibrous tail and a globular head, which binds to actin. The myosin head also binds to ATP, which is the source of energy for muscle movement. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Z-disk
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called muscle fibers or myofibers) which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long, fibrous tail and a globular head, which binds to actin. The myosin head also binds to ATP, which is the source of energy for muscle movement. My ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Striated Muscle
Striations means a series of ridges, furrows or linear marks, and is used in several ways: * Glacial striation * Striation (fatigue), in material * Striation (geology), a ''striation'' as a result of a geological fault * Striation Valley, in Antarctica * In hyperbolic geometry, a ''striation'' is a reflection across two parallel mirrors. * In anatomy, striated muscle * Striations can be found in certain glasses. These have been caused by turbulent flow during teeming (pouring) of the glass. * Striations can be observed in clouds In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid drop (liquid), droplets, ice crystals, frozen crystals, or other particulates, particles suspended in the atmosphere of a planetary body or similar space. .... See Barber's pole. * Ballistic fingerprinting {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
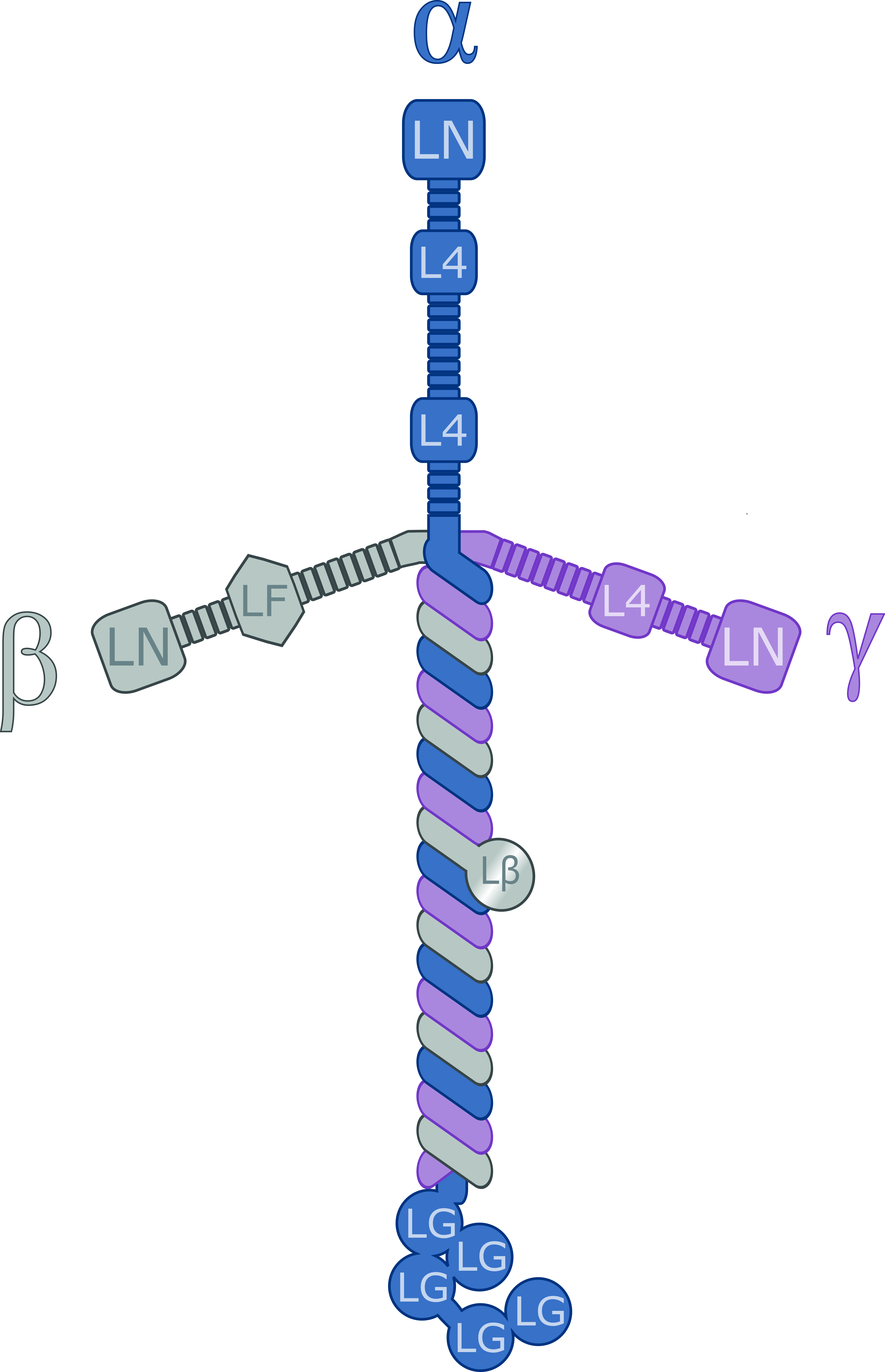
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina (one of the layers of the basement membrane), the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa). They contain three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect to form a cross-like structure that can bind to other cell membrane and extracellular matrix molecules. The three shorter arms are particularly good at binding to other laminin molecules, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystroglycan
Dystroglycan is a protein that in humans is encoded by the ''DAG1'' gene. Dystroglycan is one of the dystrophin-associated glycoproteins, which is encoded by a 5.5 kb transcript in ''Homo sapiens'' on chromosome 3. There are two exons that are separated by a large intron. The spliced exons code for a protein product that is finally cleaved into two non-covalently associated subunits, lpha(N-terminal) and eta(C-terminal). Function In skeletal muscle the dystroglycan complex works as a transmembrane linkage between the extracellular matrix and the cytoskeleton. lphadystroglycan is extracellular and binds to merosin lpha2 laminin in the basement membrane, while etadystroglycan is a transmembrane protein and binds to dystrophin, which is a large rod-like cytoskeletal protein, absent in Duchenne muscular dystrophy patients. Dystrophin binds to intracellular actin cables. In this way, the dystroglycan complex, which links the extracellular matrix to the intracellular actin ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcospan
Originally identified as Kirsten ras associated gene (krag), Sarcospan (SSPN) is a 25-kDa transmembrane protein located in the dystrophin-associated protein complex of skeletal muscle cells, where it is most abundant. It contains four transmembrane spanning helices with both N- and C-terminal domains located intracellularly. Loss of SSPN expression occurs in patients with Duchenne muscular dystrophy. Dystrophin Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the cost ... is required for proper localization of SSPN. SSPN is also an essential regulator of Akt signaling pathways. Without SSPN, Akt signaling pathways will be hindered and muscle regeneration will not occur. Sarcospan in Muscular Dystrophy The loss of dystrophin results in muscular dystrophy. SSPN upregulates the levels of Utro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcoglycan
The sarcoglycans are a family of transmembrane proteins (α, β, γ, δ or ε) involved in the protein complex responsible for connecting the muscle fibre cytoskeleton to the extracellular matrix, preventing damage to the muscle fibre sarcolemma through shearing forces. The dystrophin glycoprotein complex (DGC) is a membrane-spanning complex that links the interior cytoskeleton to the extracellular matrix in muscle. The sarcoglycan complex is a subcomplex within the DGC and is composed of six muscle-specific, transmembrane proteins (alpha-, beta-, gamma-, delta-, epsilon-,and zeta-sarcoglycan). The sarcoglycans are asparagine-linked glycosylated proteins with single transmembrane domains. The disorders caused by the mutations of the sarcoglycans are called sarcoglycanopathies. Mutations in the α, β, γ or δ genes (not ε) encoding these proteins can lead to the associated limb-girdle muscular dystrophy. Genes * SGCA * SGCB * SGCD * SGCE * SGCG * SGCZ Sarcoglycan zeta als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desmin
Desmin is a protein that in humans is encoded by the ''DES'' gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture. Structure Desmin is a 53.5 kD protein composed of 470 amino acids, encoded by the human ''DES'' gene located on the long arm of chromosome 2. There are three major domains to the desmin protein: a conserved alpha helix rod, a variable non alpha helix head, and a carboxy-terminal tail. Desmin, as all intermediate filaments, shows no polarity when assembled. The rod domain consists of 308 amino acids with parallel alpha helical coiled coil dimers and three linkers to disrupt it. The rod domain connects to the head domain. The head domain 84 amino acids with many arginine, serine, and aromatic residues is important in filament assembly and dimer-dimer interactions. The tail domain is responsible for the integration of filaments and interactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dystroglycans
Dystroglycan is a protein that in humans is encoded by the ''DAG1'' gene. Dystroglycan is one of the dystrophin-associated glycoproteins, which is encoded by a 5.5 kb transcript in ''Homo sapiens'' on chromosome 3. There are two exons that are separated by a large intron. The spliced exons code for a protein product that is finally cleaved into two non-covalently associated subunits, lpha(N-terminal) and eta(C-terminal). Function In skeletal muscle the dystroglycan complex works as a transmembrane linkage between the extracellular matrix and the cytoskeleton. lphadystroglycan is extracellular and binds to merosin lpha2 laminin in the basement membrane, while etadystroglycan is a transmembrane protein and binds to dystrophin, which is a large rod-like cytoskeletal protein, absent in Duchenne muscular dystrophy patients. Dystrophin binds to intracellular actin cables. In this way, the dystroglycan complex, which links the extracellular matrix to the intracellular actin cable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |