|
Copper(I) Cyanide
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.H. Wayne Richardson "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Structure Copper cyanide is a coordination polymer. It exists in two polymorphs both of which contain - u-CN chains made from linear copper(I) centres linked by cyanide bridges. In the high-temperature polymorph, HT-CuCN, which is isostructural with AgCN, the linear chains pack on a hexagonal lattice and adjacent chains are off set by +/- 1/3 ''c'', Figure 1. In the low-temperature polymorph, LT-CuCN, the chains deviate from linearity and pack into rippled layers which pack in an AB fashion with chains in adjacent layers rotated by 49 °, Figure 2. File:Structure of HT- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanides
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. In inorganic cyanides, the cyanide group is present as the anion . Soluble salts such as sodium cyanide (NaCN) and potassium cyanide (KCN) are highly toxic. Hydrocyanic acid, also known as hydrogen cyanide, or HCN, is a highly volatile liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts. Organic cyanides are usually called nitriles. In nitriles, the group is linked by a covalent bond to carbon. For example, in acetonitrile (), the cyanide group is bonded to methyl (). Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus rather toxic. Bonding The cyanide ion is isoelectronic with carbon monoxide and wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Compounds
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called ''cuprous'' and ''cupric'', respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes. Binary compounds As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, and halides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfide and copper(II) sulfide. Cuprous halides with fluorine, chlorine, bromine, and iodine are known, as are cupric halides with fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine. :2 Cu2+ + 4 I− → 2 CuI + I2 Coordination chemistry Copper forms coordination complexes with ligands. In aqueous solution, copper(II) exists as . This c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
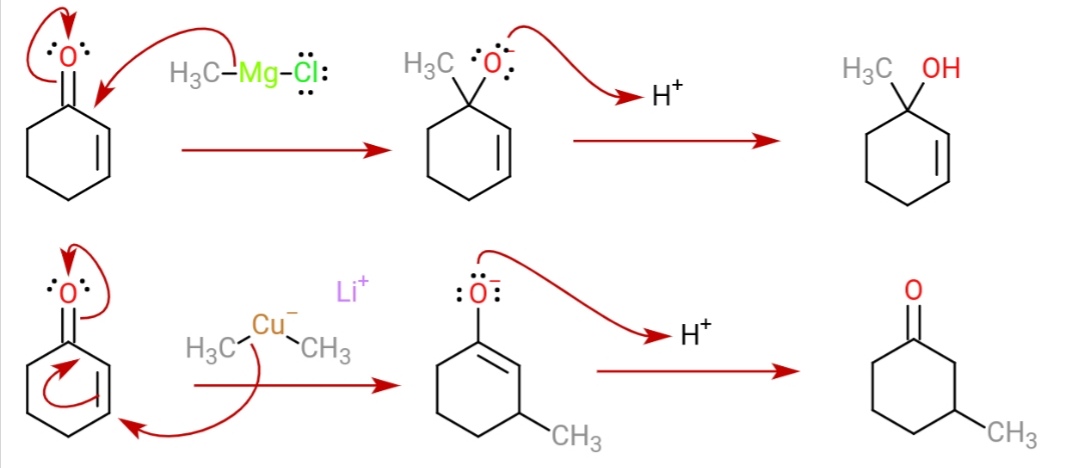
Gilman Reagent
A Gilman reagent is a lithium and copper ( diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks. Reactions These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the negative charge is trapped in a nucleophilic acyl substitution with the ester group forming a cyclic enone. Due to the softness of the nucleophile, they do 1,4 addition on conjugated enones, rather than 1,2 addition. : Structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocopper Chemistry
Organocopper compounds is the chemistry of organometallic compounds containing a carbon to copper chemical bond. Organocopper chemistry is the study of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry. The first organocopper compound, the explosive copper(I) acetylide Cu2C2 (Cu−C≡C−Cu), was synthesized by Rudolf Christian Böttger in 1859 by passing acetylene gas through a solution of copper(I) chloride: :C2H2 + 2 CuCl → Cu2C2 + 2 HCl Structure and bonding Organocopper compounds are diverse in structure and reactivity, but organocopper compounds are largely limited in oxidation states to copper(I), sometimes denoted Cu+. As a d10 metal center, it is related to Ni(0), but owing to its higher oxidation state, it engages in less pi-backbonding. Organic derivatives of Cu(II) and Cu(III) are invoked as intermediates but rarely isolated or even observed. In terms of geometry, copper(I) adopts symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper Electroplating
Copper electroplating is the process of electroplating a layer of copper onto the surface of a metal object. Copper is used both as a standalone coating and as an undercoat onto which other metals are subsequently plated. The copper layer can be decorative, provide corrosion resistance, increase electrical and thermal conductivity, or improve the adhesion of additional deposits to the substrate. Overview Copper electroplating takes place in an electrolytic cell using electrolysis. As with all plating processes, the part to be plated must be cleaned before depositing metal to remove soils, grease, oxides, and defects. After precleaning, the part is immersed in the cell's aqueous electrolyte solution and functions as the cathode. A copper anode is also immersed in the solution. During plating, a direct electric current is applied to the cell which causes the copper in the anode to dissolve into the electrolyte through oxidation, losing electrons and ionizing into copper cations. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudohalide
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms ''Ps''–''Ps'' or ''Ps''–X (where ''Ps'' is a pseudohalogen group), such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferouscyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide. Common pseudohalogens and their nomenclature Many pseudohalogens are known by specialized common names according to where they occur in a compound. Well-known ones include (the true halogen chlorine is listed for comparison): Au− is considered to be a pseudohalogen ion due to its disproportionation reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒ C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also '' Cyano radical''.) Cyanogen is the anhydride of oxamide: :H2NC(O)C(O)NH2 → NCCN + 2 H2O although oxamide is manufactured from cyanogen by hydrolysis: :NCCN + 2 H2O → H2NC(O)C(O)NH2 Preparation Cyanogen is typically generated from cyanide compounds. One laboratory method entails thermal decomposition of mercuric cyanide: :2 Hg(CN)2 → (CN)2 + Hg2(CN)2 Alternatively, one can combine solutions of copper(II) salts (such as copper(II) su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanide
Sodium cyanide is a poisonous compound with the formula Na C N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. Production and chemical properties Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide: :HCN + NaOH → NaCN + H2O Worldwide production was estimated at 500,000 tons in the year 2006. Formerly it was prepared by the Castner process involving the reaction of sodium amide with carbon at elevated temperatures. : NaNH2 + C → NaCN + H2 The structure of solid NaCN is related to that of sodium chloride. The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. When treated with acid, it forms the toxic gas hydrogen cyanide: : NaCN + H+ → HCN + Na+ Because the salt is derived from a weak acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper,Antoine-François de Fourcroy, tr. by Robert Heron (1796) "Elements of Chemistry, and Natural History: To which is Prefixed the Philosophy of Chemistry". J. Murray and others, Edinburgh. Page 348. and Roman vitriol.Oxford University Press,Roman vitriol, Oxford Living Dictionaries. Accessed on 2016-11-13 It exothermically dissolves in water to give the aquo complex , which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The centers are interconnected by sulfate anions to form chains. Anhydrous copper su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |