|
Cathodoluminescence
Cathodoluminescence is an optical and electromagnetic phenomenon in which electrons impacting on a luminescent material such as a phosphor, cause the emission of photons which may have wavelengths in the visible spectrum. A familiar example is the generation of light by an electron beam scanning the phosphor-coated inner surface of the screen of a television that uses a cathode ray tube. Cathodoluminescence is the inverse of the photoelectric effect, in which electron emission is induced by irradiation with photons. Origin Luminescence in a semiconductor results when an electron in the conduction band recombines with a hole in the valence band. The difference energy (band gap) of this transition can be emitted in form of a photon. The energy (color) of the photon, and the probability that a photon and not a phonon will be emitted, depends on the material, its purity, and the presence of defects. First, the electron has to be excited from the valence band into the conduct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathodoluminescence Microscope
A cathodoluminescence (CL) microscope combines methods from electron and regular (light optical) microscopes. It is designed to study the luminescence characteristics of polished thin sections of solids irradiated by an electron beam. Using a cathodoluminescence microscope, structures within crystals or fabrics can be made visible which cannot be seen in normal light conditions. Thus, for example, valuable information on the growth of minerals can be obtained. CL-microscopy is used in geology, mineralogy and materials science (rocks, minerals, volcanic ash, glass, ceramic, concrete, fly ash, etc.). More recently, scientists have begun to investigate its application for studying biological samples, using rare earth element-doped inorganic nanocrystals as imaging probes. Correlative Cathodoluminescence Electron Microscopy (CCLEM) can also be performed on focus ion beam (FIB) sectioned samples, hence potentially enabling 3D CCLEM. CL color and intensity are dependent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
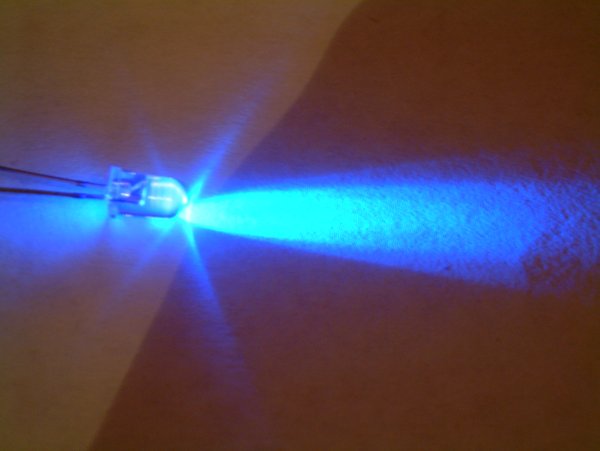
InGaN Crystal SEM%2BCL
Indium gallium nitride (InGaN, ) is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/ group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared (0.69 eV) for InN to the ultraviolet (3.4 eV) of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7. Applications LEDs Indium gallium nitride is the light-emitting layer in modern blue and green LEDs and often grown on a GaN buffer on a transparent substrate as, e.g. sapphire or silicon carbide. It has a high heat capacity and its sensitivity to ionizing radiation is low (like other group III nitrides), making it also a potentially suitable material for solar photovoltaic devices, specifically for arrays for satellites. It is theoretically predicted that spinodal decomposition of indium nitride should occur for compositions between 15% and 85%, l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond (side View)
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam ( cathode rays) in a cathode-ray tube. When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days. Fluorescent materials are used in applications in whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantization of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were pottery objects (''pots,'' ''vessels or vases'') or figurines made from clay, either by itself or mixed with other materials like silica, hardened and sintered in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as in semiconductors. The word "'' ceramic''" comes from the Greek word (), "of pottery" or "for pottery", from (), "potter's clay, tile, pottery". The earliest kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. History Early writing on mineralogy, especially on gemstones, comes from ancient Babylonia, the ancient Greco-Roman world, ancient and medieval China, and Sanskrit texts from ancient India and the ancient Islamic world. Books on the subject included the '' Naturalis Historia'' of Pliny the Elder, which not only described many different minerals but also explained many of their properties, and Kitab al Jawahir (Book of Precious Stones) by Persian scientist Al-Biruni. The German Renaissance specialist Georgius Agricola wrote works such as '' De re metallica'' (''On Metals'', 1556) and '' De Natura Fossilium' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geology
Geology () is a branch of natural science concerned with Earth and other astronomical objects, the features or rocks of which it is composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth sciences, including hydrology, and so is treated as one major aspect of integrated Earth system science and planetary science. Geology describes the structure of the Earth on and beneath its surface, and the processes that have shaped that structure. It also provides tools to determine the relative and absolute ages of rocks found in a given location, and also to describe the histories of those rocks. By combining these tools, geologists are able to chronicle the geological history of the Earth as a whole, and also to demonstrate the age of the Earth. Geology provides the primary evidence for plate tectonics, the evolutionary history of life, and the Earth's past climates. Geologists broadly study the properties and processes of E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Structure
In solid-state physics, the electronic band structure (or simply band structure) of a solid describes the range of energy levels that electrons may have within it, as well as the ranges of energy that they may not have (called ''band gaps'' or ''forbidden bands''). Band theory derives these bands and band gaps by examining the allowed quantum mechanical wave functions for an electron in a large, periodic lattice of atoms or molecules. Band theory has been successfully used to explain many physical properties of solids, such as electrical resistivity and optical absorption, and forms the foundation of the understanding of all solid-state devices (transistors, solar cells, etc.). Why bands and band gaps occur The electrons of a single, isolated atom occupy atomic orbitals each of which has a discrete energy level. When two or more atoms join together to form a molecule, their atomic orbitals overlap and hybridize. Similarly, if a large number ''N'' of identical atoms co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photons that excite electrons to a higher energy level in an atom), hence the prefix ''photo-''. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductorsHayes, G.R.; Deveaud, B. (2002). "Is Luminescence from Quantum Wells Due to Excitons?". ''Physica Status Solidi A'' 190 (3): 637–640doi:10.1002/1521-396X(200204)190:33.0.CO;2-7/ref> up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours. Observation of photoluminescence at a certain e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)