|
Carnot Method
The Carnot method is an allocation procedure for dividing up fuel input (primary energy, end energy) in joint production processes that generate two or more energy products in one process (e.g. cogeneration or trigeneration). It is also suited to allocate other streams such as CO2-emissions or variable costs. The potential to provide physical work (exergy) is used as the distribution key. For heat this potential can be assessed the Carnot efficiency. Thus, the Carnot method is a form of an exergetic allocation method. It uses mean heat grid temperatures at the output of the process as a calculation basis. The Carnot method's advantage is that no external reference values are required to allocate the input to the different output streams; only endogenous process parameters are needed. Thus, the allocation results remain unbiased of assumptions or external reference values that are open for discussion. Fuel allocation factor The fuel share ael which is needed to generate the comb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Energy
Primary energy (PE) is an energy form found in nature that has not been subjected to any human engineered conversion process. It is energy contained in raw fuels, and other forms of energy, including waste, received as input to a system. Primary energy can be non-renewable or renewable. Where primary energy is used to describe fossil fuels, the embodied energy of the fuel is available as thermal energy and around 70% is typically lost in conversion to electrical or mechanical energy. There is a similar 60-80% conversion loss when solar and wind energy is converted to electricity, but today's UN conventions on energy statistics counts the electricity made from wind and solar as the primary energy itself for these sources. One consequence of this counting method is that the contribution of wind and solar energy is under reported compared to fossil energy sources, and there is hence an international debate on how to count primary energy from wind and solar. Primary energy is u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
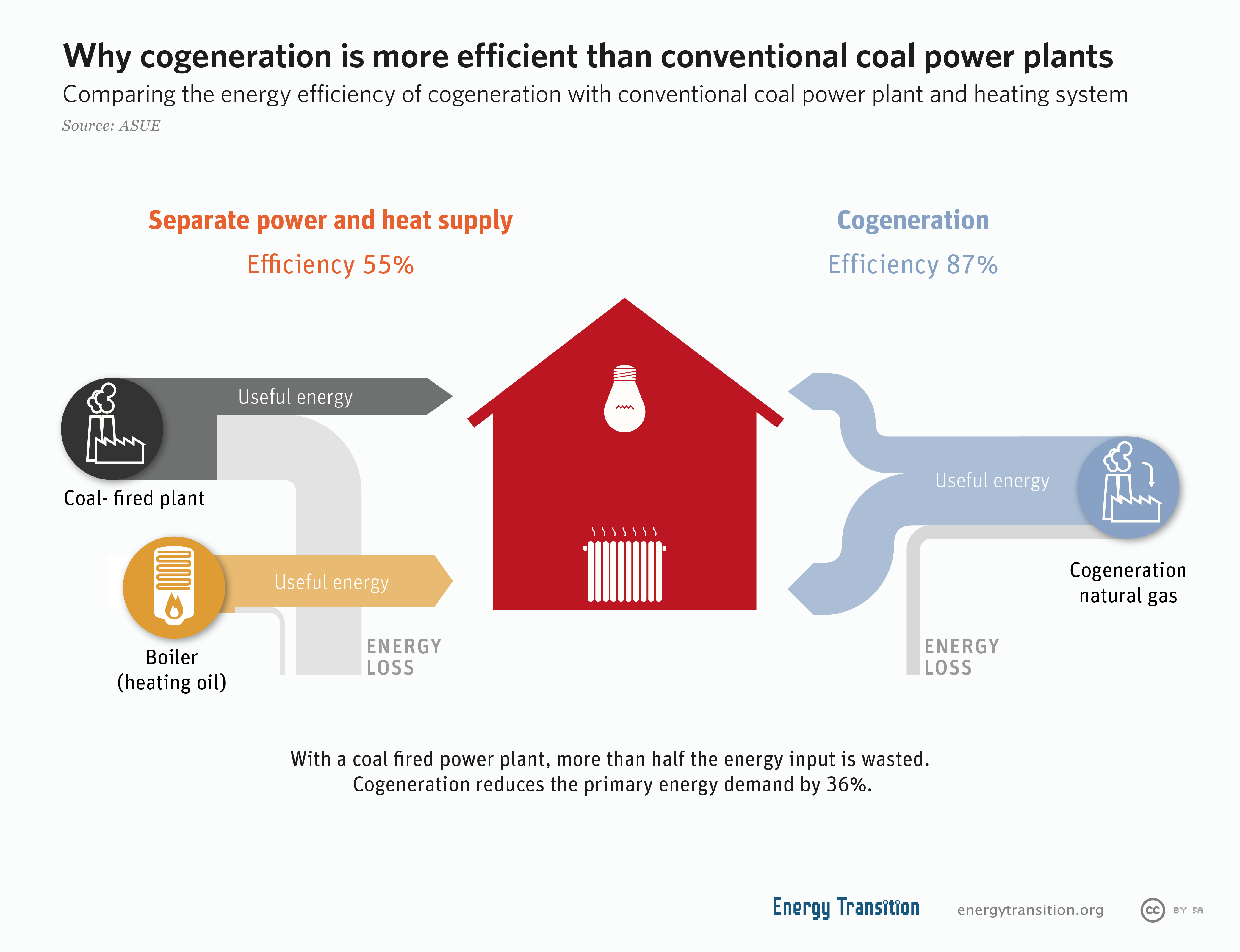
Cogeneration
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Cogeneration is a more efficient use of fuel or heat, because otherwise- wasted heat from electricity generation is put to some productive use. Combined heat and power (CHP) plants recover otherwise wasted thermal energy for heating. This is also called combined heat and power district heating. Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling. The supply of high-temperature heat first drives a gas or steam turbine-powered generator. The resulting low-temperature waste heat is then used for water or space heating. At smaller scales (typically below 1 MW), a gas engine or diesel engine may be used. Cogeneration is also common with geothermal power plants as they often produce relatively ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CO2 Emissions
Greenhouse gas emissions from human activities strengthen the greenhouse effect, contributing to climate change. Most is carbon dioxide from burning fossil fuels: coal, oil, and natural gas. The largest emitters include coal in China and large oil and gas companies, many state-owned by OPEC and Russia. Human-caused emissions have increased atmospheric carbon dioxide by about 50% over pre-industrial levels. The growing levels of emissions have varied, but it was consistent among all greenhouse gases (GHG). Emissions in the 2010s averaged 56 billion tons a year, higher than ever before. Electricity generation and transport are major emitters; the largest single source, according to the United States Environmental Protection Agency, is transportation, accounting for 27% of all USA greenhouse gas emissions. Deforestation and other changes in land use also emit carbon dioxide and methane. The largest source of anthropogenic methane emissions is agriculture, closely followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exergy
In thermodynamics, the exergy of a system is the maximum useful work possible during a process that brings the system into equilibrium with a heat reservoir, reaching maximum entropy. When the surroundings are the reservoir, exergy is the potential of a system to cause a change as it achieves equilibrium with its environment. Exergy is the energy that is available to be used. After the system and surroundings reach equilibrium, the exergy is zero. Determining exergy was also the first goal of thermodynamics. The term "exergy" was coined in 1956 by Zoran Rant (1904–1972) by using the Greek '' ex'' and ''ergon'' meaning "from work", but the concept had been earlier developed by J Willard Gibbs (the namesake of Gibbs free energy) in 1873. Energy is neither created nor destroyed during a process. Energy changes from one form to another (''see First Law of Thermodynamics''). In contrast, exergy is always destroyed when a process is irreversible, for example loss of heat to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Efficiency
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is reversible, and there is no generation of entropy. (In other words, entropy is conserved; entropy is only transferred between the thermal reservoirs and the system without gain or loss of it.) When work is applied to the system, heat moves from the cold to hot reser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laws Of Thermodynamics
The laws of thermodynamics are a set of scientific laws which define a group of physical quantities, such as temperature, energy, and entropy, that characterize thermodynamic systems in thermodynamic equilibrium. The laws also use various parameters for thermodynamic processes, such as thermodynamic work and heat, and establish relationships between them. They state empirical facts that form a basis of precluding the possibility of certain phenomena, such as perpetual motion. In addition to their use in thermodynamics, they are important fundamental laws of physics in general, and are applicable in other natural sciences. Traditionally, thermodynamics has recognized three fundamental laws, simply named by an ordinal identification, the first law, the second law, and the third law.Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Kittel, C. Kroemer, H. (1980). ''Thermal Physics'', second edi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water (data Page)
This page provides supplementary data to the article properties of water. Further comprehensive authoritative data can be found at thNIST Webbookpage on thermophysical properties of fluids. Structure and properties Thermodynamic properties Liquid physical properties Water/steam equilibrium properties Vapor pressure formula for steam in equilibrium with liquid water: : \log_ P = A - \frac, where ''P'' is equilibrium vapor pressure in k Pa, and ''T'' is temperature in kelvins. For ''T'' = 273 K to 333 K: ''A'' = 7.2326; ''B'' = 1750.286; ''C'' = 38.1. For ''T'' = 333 K to 423 K: ''A'' = 7.0917; ''B'' = 1668.21; ''C'' = 45.1. Data in the table above is given for water–steam equilibria at various temperatures over the entire temperature range at which liquid water can exist. Pressure of the equilibrium is given in the second column in k Pa. The third column is the heat content of each gram of the liquid phase relative to water at 0 °C. The fourth column is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot decr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waste Heat
Waste heat is heat that is produced by a machine, or other process that uses energy, as a byproduct of doing work. All such processes give off some waste heat as a fundamental result of the laws of thermodynamics. Waste heat has lower utility (or in thermodynamics lexicon a lower exergy or higher entropy) than the original energy source. Sources of waste heat include all manner of human activities, natural systems, and all organisms, for example, incandescent light bulbs get hot, a refrigerator warms the room air, a building gets hot during peak hours, an internal combustion engine generates high-temperature exhaust gases, and electronic components get warm when in operation. Instead of being "wasted" by release into the ambient environment, sometimes waste heat (or cold) can be used by another process (such as using hot engine coolant to heat a vehicle), or a portion of heat that would otherwise be wasted can be reused in the same process if make-up heat is added to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cogeneration
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Cogeneration is a more efficient use of fuel or heat, because otherwise- wasted heat from electricity generation is put to some productive use. Combined heat and power (CHP) plants recover otherwise wasted thermal energy for heating. This is also called combined heat and power district heating. Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling. The supply of high-temperature heat first drives a gas or steam turbine-powered generator. The resulting low-temperature waste heat is then used for water or space heating. At smaller scales (typically below 1 MW), a gas engine or diesel engine may be used. Cogeneration is also common with geothermal power plants as they often produce relatively ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Variable Cost
Variable costs are costs that change as the quantity of the good or service that a business produces changes.Garrison, Noreen, Brewer. Ch 2 - Managerial Accounting and Costs Concepts, pp 48 Variable costs are the sum of marginal costs over all units produced. They can also be considered normal costs. Fixed costs and variable costs make up the two components of total cost. Direct costs are costs that can easily be associated with a particular cost object.Garrison, Noreen, Brewer. Ch 2 - Managerial Accounting and Costs Concepts, pp 51 However, not all variable costs are direct costs. For example, variable manufacturing overhead costs are variable costs that are indirect costs, not direct costs. Variable costs are sometimes called unit-level costs as they vary with the number of units produced. Direct labor and overhead are often called conversion cost,Garrison, Noreen, Brewer. Ch 2 - Managerial Accounting and Costs Concepts, pp 39 while direct material and direct labor are often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)