|
Carbonaceous
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. The atoms of carbon can bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through beta decay. A gra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
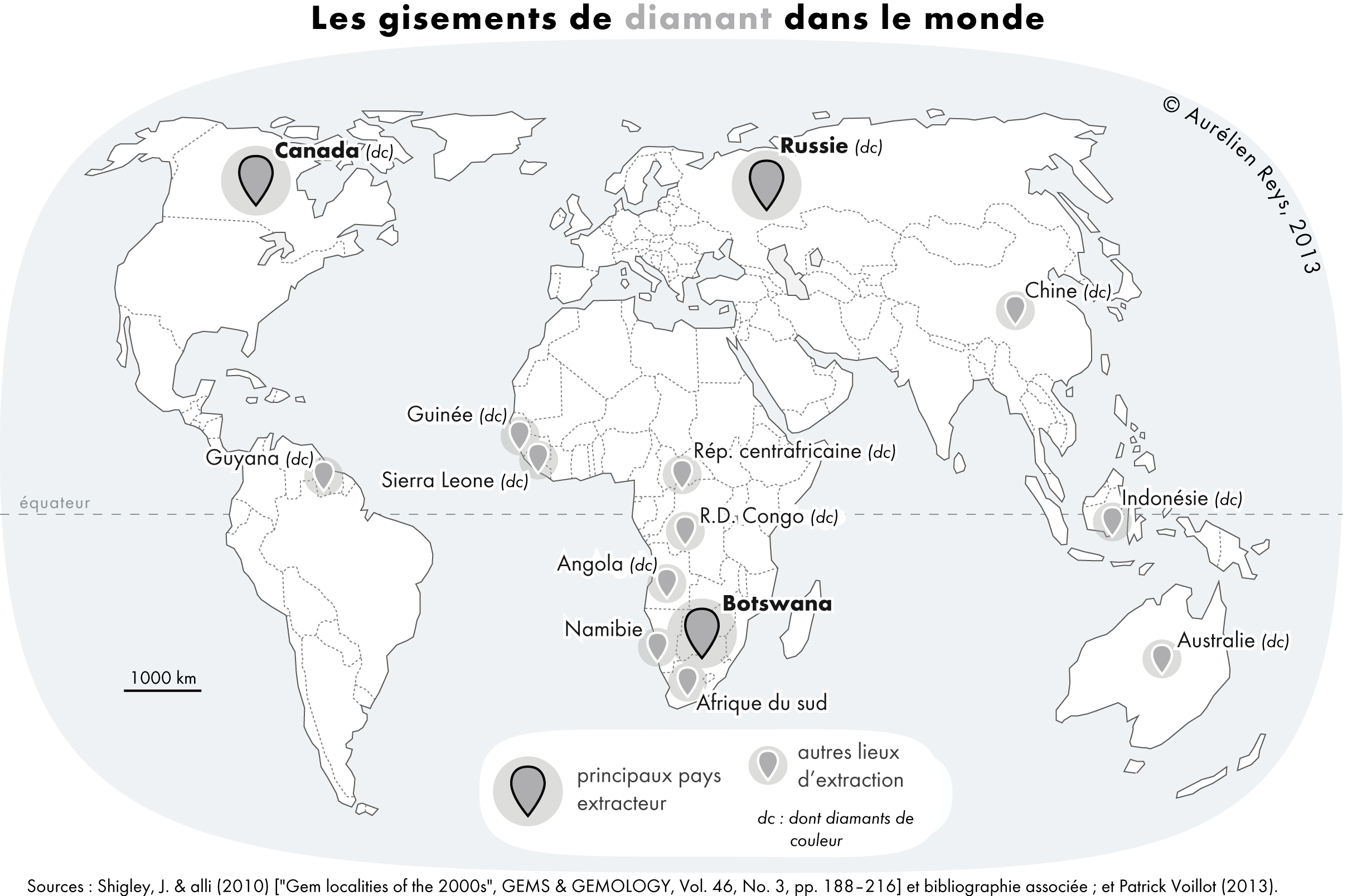
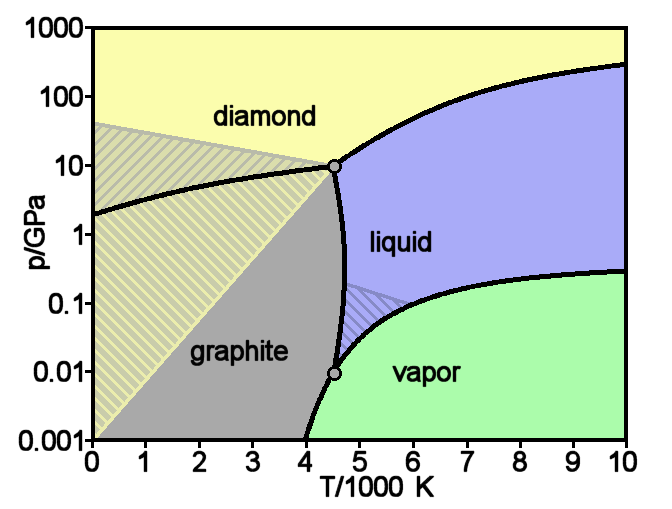
Diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) color diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a ve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abundance Of The Chemical Elements
The abundance of the chemical elements is a measure of the occurrence of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by the mass-fraction (the same as weight fraction); by the mole-fraction (fraction of atoms by numerical count, or sometimes fraction of molecules in gases); or by the volume-fraction. Volume-fraction is a common abundance measure in mixed gases such as planetary atmospheres, and is similar in value to molecular mole-fraction for gas mixtures at relatively low densities and pressures, and ideal gas mixtures. Most abundance values in this article are given as mass-fractions. For example, the abundance of oxygen in pure water can be measured in two ways: the ''mass fraction'' is about 89%, because that is the fraction of water's mass which is oxygen. However, the ''mole-fraction'' is about 33% because only 1 atom of 3 in water, H2O, is oxygen. As another example, looking at the ''mass-f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular cryst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropy
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are bonded together in a different manner. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a cubic lattice of tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for compounds. The more general term, used for any compound, is polymorphism, although its use is usually restricted to solid materials such as crystals. Allotropy refers only to different forms of an element within the same physical phase (the state of matter, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Carbon
Carbon is capable of forming many allotropes (structurally different forms of the same element) due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Diamond Diamond is a well-known allotrope of carbon. The hardness, extremely high refractive index, and high dispersion of light make diamond useful for industrial applications and for jewelry. Diamond is the hardest known natural mineral. This makes it an excellent abrasive and makes it hold polish and luster extre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Education
Pearson Education is a British-owned education publishing and assessment service to schools and corporations, as well for students directly. Pearson owns educational media brands including Addison–Wesley, Peachpit, Prentice Hall, eCollege, Longman, Scott Foresman, and others. Pearson is part of Pearson plc, which formerly owned the ''Financial Times''. It claims to have been formed in 1840, with the current incarnation of the company created when Pearson plc purchased the education division of Simon & Schuster (including Prentice Hall and Allyn & Bacon) from Viacom and merged it with its own education division, Addison-Wesley Longman, to form Pearson Education. Pearson Education was rebranded to Pearson in 2011 and split into an International and a North American division. Although Pearson generates approximately 60 percent of its sales in North America, it operates in more than 70 countries. Pearson International is headquartered in London, and maintains offices across ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Body
The human body is the structure of a human being. It is composed of many different types of cells that together create tissues and subsequently organ systems. They ensure homeostasis and the viability of the human body. It comprises a head, hair, neck, trunk (which includes the thorax and abdomen), arms and hands, legs and feet. The study of the human body involves anatomy, physiology, histology and embryology. The body varies anatomically in known ways. Physiology focuses on the systems and organs of the human body and their functions. Many systems and mechanisms interact in order to maintain homeostasis, with safe levels of substances such as sugar and oxygen in the blood. The body is studied by health professionals, physiologists, anatomists, and by artists to assist them in their work. Composition The human body is composed of elements including hydrogen, oxygen, carbon, calcium and phosphorus. These elements reside in trillions of cells and non-cellula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-based Life
{{disambiguation ...
Carbon-based may refer to: * Biology * based on Carbon * Carbon-based life * Carbon chauvinism Carbon chauvinism is a neologism meant to disparage the assumption that the chemical processes of hypothetical extraterrestrial life must be constructed primarily from carbon (organic compounds) because as far as we know, carbon's chemical and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surface is made up of the ocean, dwarfing Earth's polar ice, lakes, and rivers. The remaining 29% of Earth's surface is land, consisting of continents and islands. Earth's surface layer is formed of several slowly moving tectonic plates, which interact to produce mountain ranges, volcanoes, and earthquakes. Earth's liquid outer core generates the magnetic field that shapes the magnetosphere of the Earth, deflecting destructive solar winds. The atmosphere of the Earth consists mostly of nitrogen and oxygen. Greenhouse gases in the atmosphere like carbon dioxide (CO2) trap a part of the energy from the Sun close to the surface. Water vapor is widely present in the atmosphere and forms clouds that cover most of the planet. More solar e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |