|
Candidalysin
Candidalysin is a cytolytic 31-amino acid α-helical amphipathic peptide toxin secreted by the opportunistic pathogen ''Candida albicans''. This toxin is a fungal example of a classical virulence factor. Hyphal morphogenesis in ''C. albicans'' is associated with damage to host epithelial cells; during this process Candidalysin is released and intercalates in host membranes. Candidalysin promotes damage of oral epithelial cells and induces lactate dehydrogenase release and calcium ion influx. It is unique in the fact that it is the first peptide toxin to be identified in any human fungal pathogen. Candidalysin is a product of the larger protein Ece1 (extent of cell elongation 1). Sequential processing of Ece1 at lysine/arginine residues by the proteases Kex2 and Kex1 releases several peptides, including the toxin Candidalysin. Consequently, Candidalysin is also known as Ece1-III62–92K. ''C. albicans s''trains missing Candidalysin do not damage epithelial cells and are said to b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Candida Albicans
''Candida albicans'' is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also survive outside the human body. It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults. It is usually a commensal organism, but it can become pathogenic in immunocompromised individuals under a variety of conditions. It is one of the few species of the genus '' Candida'' that causes the human infection candidiasis, which results from an overgrowth of the fungus. Candidiasis is, for example, often observed in HIV-infected patients. ''C. albicans'' is the most common fungal species isolated from biofilms either formed on (permanent) implanted medical devices or on human tissue. ''C. albicans'', ''C. tropicalis'', ''C. parapsilosis'', and ''C. glabrata'' are together responsible for 50–90% of all cases of candidiasis in humans. A mortality rate of 40% has been reported for patients with systemic candidiasis due to ''C. albicans' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Virulence Factor
Virulence factors (preferably known as pathogenicity factors or effectors in plant science) are cellular structures, molecules and regulatory systems that enable microbial pathogens (bacteria, viruses, fungi, and protozoa) to achieve the following: * colonization of a niche in the host (this includes movement towards and attachment to host cells) * immunoevasion, evasion of the host's immune response * immunosuppression, inhibition of the host's immune response (this includes leukocidin-mediated cell death) * entry into and exit out of cells (if the pathogen is an intracellular one) * obtain nutrition from the host Specific pathogens possess a wide array of virulence factors. Some are chromosomally encoded and intrinsic to the bacteria (e.g. capsules and endotoxin), whereas others are obtained from mobile genetic elements like plasmids and bacteriophages (e.g. some exotoxins). Virulence factors encoded on mobile genetic elements spread through horizontal gene transfer, and ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytolysis
Cytolysis, or osmotic lysis, occurs when a cell bursts due to an osmotic imbalance that has caused excess water to diffuse into the cell. Water can enter the cell by diffusion through the cell membrane or through selective membrane channels called aquaporins, which greatly facilitate the flow of water. It occurs in a hypotonic environment, where water moves into the cell by osmosis and causes its volume to increase to the point where the volume exceeds the membrane's capacity and the cell bursts. The presence of a cell wall prevents the membrane from bursting, so cytolysis only occurs in animal and protozoa cells which do not have cell walls. The reverse process is plasmolysis. In bacteria Osmotic lysis would be expected to occur when bacterial cells are treated with a hypotonic solution with added lysozyme, which destroys the bacteria's cell walls. Prevention Different cells and organisms have adapted different ways of preventing cytolysis from occurring. For example, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitogen-activated Protein Kinase
A mitogen-activated protein kinase (MAPK or MAP kinase) is a type of protein kinase that is specific to the amino acids serine and threonine (i.e., a serine/threonine-specific protein kinase). MAPKs are involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines. They regulate cell functions including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis. MAP kinases are found in eukaryotes only, but they are fairly diverse and encountered in all animals, fungi and plants, and even in an array of unicellular eukaryotes. MAPKs belong to the CMGC (CDK/MAPK/GSK3/CLK) kinase group. The closest relatives of MAPKs are the cyclin-dependent kinases (CDKs). Discovery The first mitogen-activated protein kinase to be discovered was ERK1 ( MAPK3) in mammals. Since ERK1 and its close relative ERK2 ( MAPK1) are both involved in growth factor signaling, the family ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toll-like Receptor
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles (because they are sensors of nucleic acids). TLRs received their name from their similarity to the protein coded by the toll gene. Function The ability of the immune system to re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 10
Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti- inflammatory cytokine. In humans, interleukin 10 is encoded by the ''IL10'' gene. IL-10 signals through a receptor complex consisting of two IL-10 receptor-1 and two IL-10 receptor-2 proteins. Consequently, the functional receptor consists of four IL-10 receptor molecules. IL-10 binding induces STAT3 signalling via the phosphorylation of the cytoplasmic tails of IL-10 receptor 1 + IL-10 receptor 2 by JAK1 and Tyk2 respectively. Gene and protein structure The IL-10 protein is a homodimer; each of its subunits is 178-amino-acid long. IL-10 is classified as a class-2 cytokine, a set of cytokines including IL-19, IL-20, IL-22, IL-24 (Mda-7), IL-26 and interferons type-I (IFN-alpha, -beta, -epsilon, -kappa, -omega), type-II (IFN-gamma) and type-III (IFN-lambda, also known as IL-28A, IL-28B, and IL-29). Expression and synthesis In humans, IL-10 is encoded by the ''IL10'' gene, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAPK/ERK Pathway
The MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. The signal starts when a signaling molecule binds to the receptor on the cell surface and ends when the DNA in the nucleus expresses a protein and produces some change in the cell, such as cell division. The pathway includes many proteins, such as mitogen-activated protein kinases (MAPKs), originally called extracellular signal-regulated kinases (ERKs), which communicate by adding phosphate groups to a neighboring protein ( phosphorylating it), thereby acting as an "on" or "off" switch. When one of the proteins in the pathway is mutated, it can become stuck in the "on" or "off" position, a necessary step in the development of many cancers. In fact, components of the MAPK/ERK pathway were first discovered in cancer cells, and drugs that reverse the "on" or "off" switch are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-Jun N-terminal Kinases
c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. They belong to the mitogen-activated protein kinase family, and are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock. They also play a role in T cell differentiation and the cellular apoptosis pathway. Activation occurs through a dual phosphorylation of threonine (Thr) and tyrosine (Tyr) residues within a Thr-Pro-Tyr motif located in kinase subdomain VIII. Activation is carried out by two MAP kinase kinases, MKK4 and MKK7, and JNK can be inactivated by Ser/Thr and Tyr protein phosphatases. It has been suggested that this signaling pathway contributes to inflammatory responses in mammals and insects. Isoforms The c-Jun N-terminal kinases consist of ten isoforms derived from three genes: JNK1 (four isoforms), JNK2 (four isoforms) and JNK3 (two iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
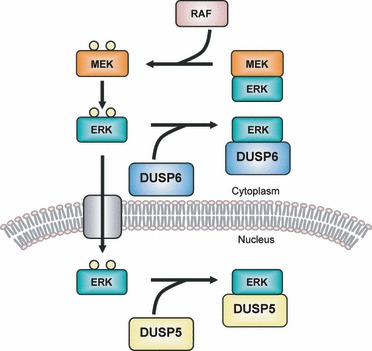
MAPK Phosphatase
MAPK phosphatases (MKPs) are the largest class of phosphatases involved in down-regulating Mitogen-activated protein kinases (MAPK) signaling. MAPK signalling pathways regulate multiple features of development and homeostasis. This can involve gene regulation, cell proliferation, programmed cell death and stress responses. MAPK phosphatases are therefore important regulator components of these pathways. Function MAPK phosphatases are only found in eukaryotes and negatively regulate MAP kinases to act as negative feedback. MKPs are also known as dual-specificity phosphatases (DUSPs) because they deactivate MAPK by dephosphorylating the Threonine and the Tyrosine residues residing in MAPKs activation site. MKPs have a catalytic region at their C-terminus and a regulatory region at their N-terminus. The position where the MAPK binds to MKP is found near the N-terminus of MKP. The binding is due to the electrostatic interactions of the positively charged residues on the MKP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AP-1 Transcription Factor
Activator protein 1 (AP-1) is a transcription factor that regulates gene expression in response to a variety of stimuli, including cytokines, growth factors, stress, and bacterial and viral infections. AP-1 controls a number of cellular processes including differentiation, proliferation, and apoptosis. The structure of AP-1 is a heterodimer composed of proteins belonging to the c-Fos, c-Jun, ATF and JDP families. History AP-1 was first discovered as a TPA-activated transcription factor that bound to a cis-regulatory element of the human metallothionein IIa ( hMTIIa) promoter and SV40. The AP-1 binding site was identified as the 12-O-Tetradecanoylphorbol-13-acetate ( TPA) response element (TRE) with the consensus sequence 5’-TGA G/C TCA-3’. The AP-1 subunit Jun was identified as a novel oncoprotein of avian sarcoma virus, and Fos-associated p39 protein was identified as the transcript of the cellular Jun gene. Fos was first isolated as the cellular homologu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P38 Mitogen-activated Protein Kinases
p38 mitogen-activated protein kinases are a class of mitogen-activated protein kinases (MAPKs) that are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis and autophagy. Persistent activation of the p38 MAPK pathway in muscle satellite cells (muscle stem cells) due to ageing, impairs muscle regeneration. p38 MAP Kinase (MAPK), also called RK or CSBP (Cytokinin Specific Binding Protein), is the mammalian orthologue of the yeast Hog1p MAP kinase, which participates in a signaling cascade controlling cellular responses to cytokines and stress. Four p38 MAP kinases, p38-α ( MAPK14), -β (MAPK11), -γ (MAPK12 / ERK6), and -δ ( MAPK13 / SAPK4), have been identified. Similar to the SAPK/JNK pathway, p38 MAP kinase is activated by a variety of cellular stresses including osmotic shock, inflammatory cytokines, lipopolysaccharides (LPS), ultraviolet light, and growth factors. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kex2 Endopeptidase
Kexin () is a prohormone-processing protease, specifically a yeast serine peptidase, found in the budding yeast (''S. cerevisiae''). It catalyzes the cleavage of -Lys-Arg- and -Arg-Arg- bonds to process yeast alpha-factor pheromone and killer toxin precursors. The human homolog is PCSK4. It is a family of subtilisin-like peptidases. Even though there are a few prokaryote kexin-like peptidases, all kexins are eukaryotes. The enzyme is encoded by the yeast gene ''KEX2'', and usually referred to in the scientific community as Kex2p. It shares structural similarities with the bacterial protease subtilisin. The first mammalian homologue of this protein to be identified was furin. In the mammal, kexin-like peptidases function in creating and regulating many differing proproteins. Nomenclature The enzyme is also known as yeast KEX2 protease, proteinase yscF, prohormone-processing endoprotease, paired-basic endopeptidase, yeast cysteine proteinase F, paired-basic endopeptidase, andren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |