|
CELA3B
Chymotrypsin-like elastase family member 3B also known as elastase-3B, protease E, or fecal elastase is an enzyme that in humans is encoded by the ''CELA3B'' gene. Clinical literature that describes human elastase 1 activity in the pancreas or fecal material is actually referring to chymotrypsin-like elastase family member 3B (i.e. the enzyme / protein this article focuses on). Function Elastases form a subfamily of serine proteases that hydrolyze many proteins in addition to elastin. Humans have six elastase genes which encode the structurally similar proteins elastase 1, 2, 2A, 2B, 3A, and 3B. Unlike other elastases, elastase 3B has little elastolytic activity. Like most of the human elastases, elastase 3B is secreted from the pancreas as a zymogen and, like other serine proteases such as trypsin, chymotrypsin and kallikrein, it has a digestive function in the intestine. Elastase 3B preferentially cleaves proteins after alanine residues. Elastase 3B may also function in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CELA1
Chymotrypsin-like elastase family member 1 (CELA1) also known as elastase-1 (ELA1) is an enzyme that in humans is encoded by the CELA1 gene. Elastases form a subfamily of serine proteases that hydrolyze many proteins in addition to elastin. Humans have six elastase genes which encode the structurally similar proteins elastase 1, 2, 2A, 2B, 3A, and 3B. Tissue distribution Elastase-1 was formerly designated pancreatic elastase 1. However unlike other elastases, pancreatic elastase 1 is not expressed in the pancreas. Hence this enzyme has been renamed as elastase-1. To date, elastase 1 expression has only been detected in skin keratinocytes. Literature that describes human elastase 1 activity in the pancreas or fecal material is actually referring to chymotrypsin-like elastase family, member 3B CELA3B). Clinical significance This enzyme has been linked to chronic pancreatitis . References Further reading * * * * * * * * External links * The MEROPS MEROPS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastase
In molecular biology, elastase is an enzyme from the class of ''proteases (peptidases)'' that break down proteins. In particular, it is a serine protease. Forms and classification Eight human genes exist for elastase: Some bacteria (including ''Pseudomonas aeruginosa'') also produce elastase. In bacteria, elastase is considered a virulence factor. Function Elastase breaks down elastin, an elastic fibre that, together with collagen, determines the mechanical properties of connective tissue. The neutrophil form breaks down the ''Outer membrane protein A'' (OmpA) of '' E. coli'' and other Gram-negative bacteria. Elastase also has the important immunological role of breaking down Shigella virulence factors. This is accomplished through the cleavage of peptide bonds in the target proteins. The specific peptide bonds cleaved are those on the carboxyl side of small, hydrophobic amino acids such as glycine, alanine, and valine. For more on how this is accomplished, see serine p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kallikrein
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein (encoded by '' KLKB1 gene'') has no known paralogue, while tissue kallikrein-related peptidases (''KLKs'') encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation. Occurrence In 1934, Eugen Werle reported finding a substance in the pancreas of humans and various animals in such large amounts that the pancreas could be taken for its site of origin. He named it kallikrein, by derivation from the Greek word for pancreas. Since then, similar enzymes have been found in the biological fluids of humans and other mammals, as well as in some snake venoms. Venom The caterpillar kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand (commonly a protein) in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries. In the most simple form of an ELISA, antigens from the sample to be tested are attached to a surface. Then, a matching antibody is applied over the surface so it can bind the antigen. This antibody is linked to an enzyme and then any unbound antibodies are removed. In the final step, a substance containing the enzyme's substrate is added. If there was binding, the subsequent reaction produces a detectable signal, most commonly a color change. Performing an ELISA involves at le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feces
Feces ( or faeces), known colloquially and in slang as poo and poop, are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the large intestine. Feces contain a relatively small amount of metabolic waste products such as bacterially altered bilirubin, and dead epithelial cells from the lining of the gut. Feces are discharged through the anus or cloaca during defecation. Feces can be used as fertilizer or soil conditioner in agriculture. They can also be burned as fuel or dried and used for construction. Some medicinal uses have been found. In the case of human feces, fecal transplants or fecal bacteriotherapy are in use. Urine and feces together are called excreta. Skatole is the principal compound responsible for the unpleasant smell of feces. Characteristics The distinctive odor of feces is due to skatole, and thiols (sulfur-containing compounds), as well as amines and carboxylic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', and '' volume concentration''. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants, such as normal concentration and osmotic concentration. Etymology The term concentration comes from the word concentrate, from the French , from con– + center, meaning “to put at the center”. Qualitative description Often in informal, non-technical language, concentration is described in a qualitative way, through the use of adjectives such as "dilute" for solutions of relatively low concentration and "concentrated" for solutions of relatively high concentration. To concentrate a solution, one must add more solute (for exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membranes. When chemically isolated, it is a yellowish crystalline solid. Cholesterol also serves as a precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Cholesterol is the principal sterol synthesized by all animals. In vertebrates, hepatic cells typically produce the greatest amounts. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as '' Mycoplasma'', which require cholesterol for growth. François Poulletier de la Salle first identified cholesterol in solid form in gallstones in 1769. However, it was not until 1815 that chemist Michel Eugène Chevreul named the compound "cholesterine". Etymology The word "cholesterol" comes from the Ancient Greek ''chole- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
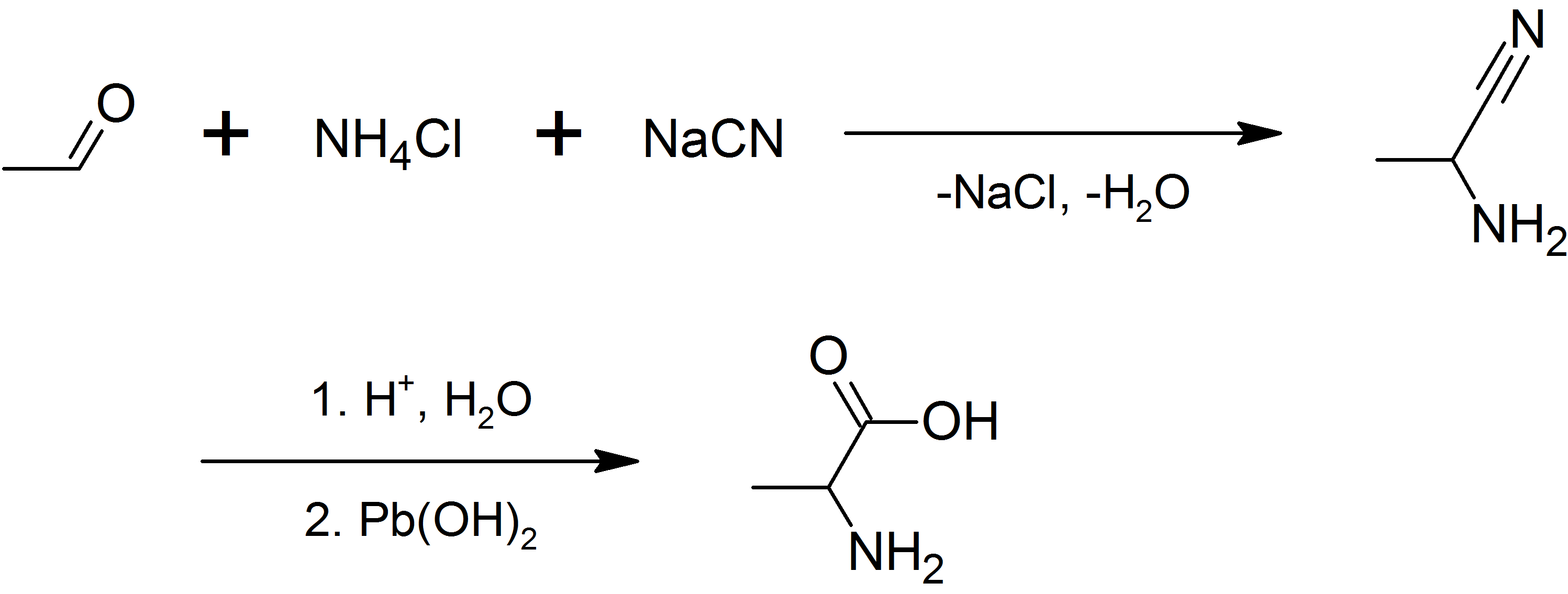
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydroly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chymotrypsin
Chymotrypsin (, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |