|
Organopalladium Compounds
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions. Organopalladium chemistry timeline * 1873 - A. N. Zaitsev reports reduction of benzophenone over palladium with hydrogen. * 1894 - Francis Phillips reports that palladium(II) chloride reduces to palladium metal by contact with ethylene. * 1907 - Autoclave technology introduced by Vladimir Ipatieff makes it possible to carry out high pressure hydrogenation. * 1956 - In the Wacker process ethylene and oxygen react to acetaldehyde with catalyst PdCl2/CuCl2. During process development, Walter Hafner also identifies the first allylpalladium complex.''Acetaldehyde from Ethylene — A Retrosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (Metal carbonyl, metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganics, metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
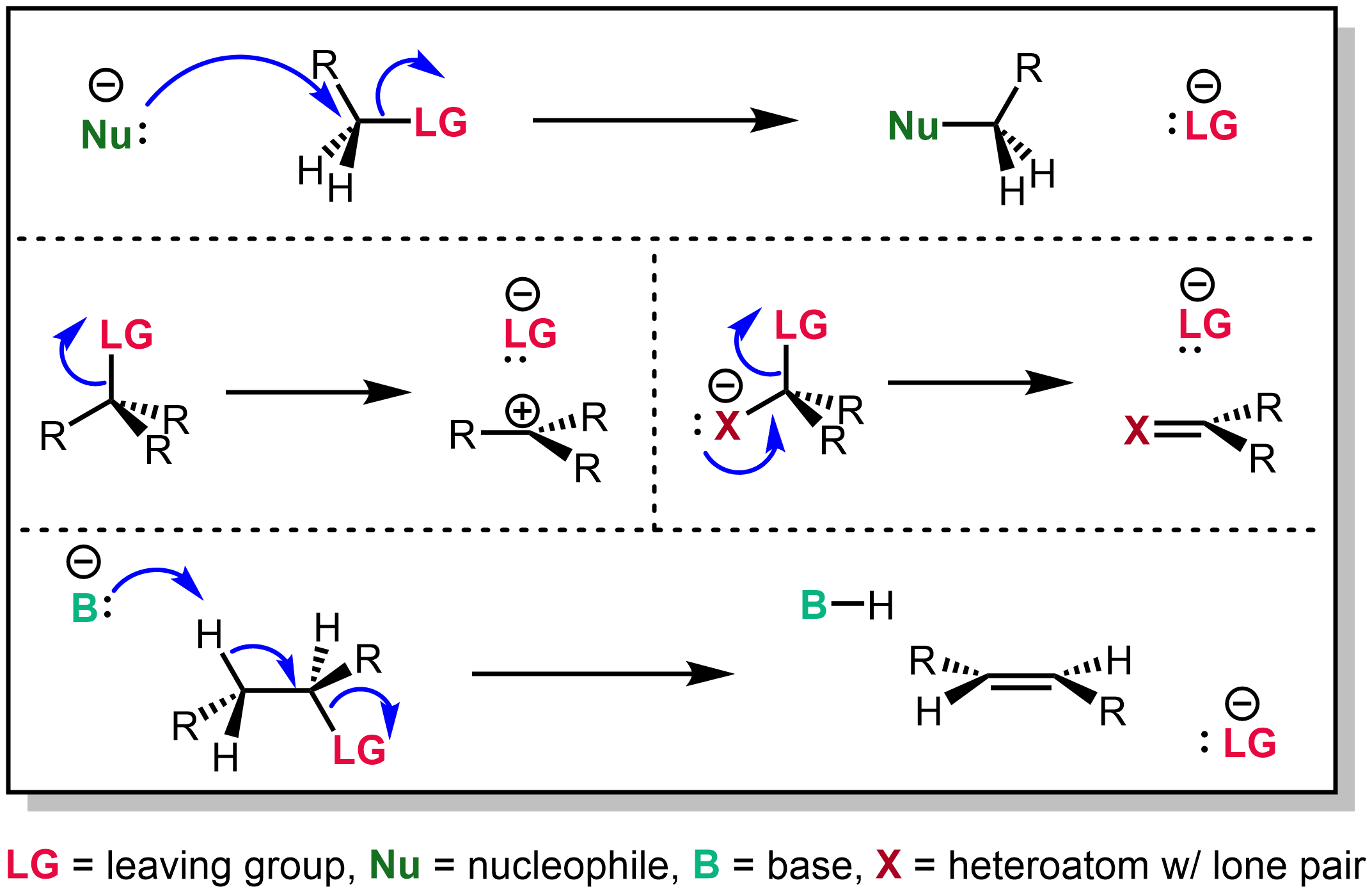
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a Substrate (chemistry), substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic Carbon–hydrogen bond, C−H bonds are about 15% weaker than the C−H bonds in ordinary Orbital hybridisation, sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylpalladium Chloride Dimer
Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula hapticity.html" ;"title="hapticity">η3-C3H5)PdClsub>2. This yellow air-stable compound is an important catalyst used in organic synthesis.Tatsuno, Y.; Yoshida, T.; Otsuka, S. "(η3-allyl)palladium(II) Complexes" Inorganic Syntheses, 1990, volume 28, pages 342-345. It is one of the most widely used transition metal allyl complexes. Structure The compound has a dimeric structure that is centrosymmetric. Each allyl group lies in a plane at an angle of about 111.5° to the square formed by the palladium and carbon atoms, and the Pd–C distances are all equal. Its unit cell is monoclinic. Synthesis The compound is prepared by purging carbon monoxide through a methanolic aqueous solution of sodium tetrachloropalladate (prepared from palladium(II) chloride and sodium chloride), and allyl chloride. :2 Na2PdCl4 + 2 CH2=CHCH2Cl + 2 CO + 2 H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: : Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Overall, decarboxylation depends upon stability of the carbanion synthon , although the anion may not be a true chemical intermediate. Typically, carboxylic acids decarboxylate slowly, but carboxylic acids with an α el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
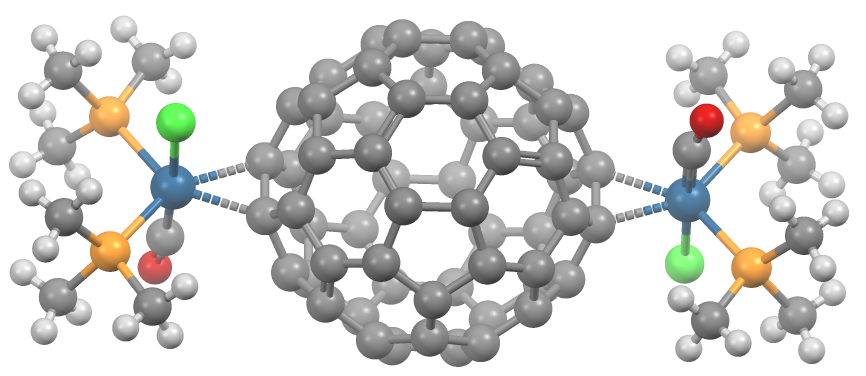
Fullerene Ligands
A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60. One year after it was prepared in milligram quantities in 1990, C60 was shown to function as a ligand in the complex [Ph3P]2Pt(η2-C60). Since this report, a variety of transition metals and binding modes were demonstrated. Most transition metal fullerene complex are derived from C60, although other fullerenes also coordinate to metals as seen with C70Rh(H)(CO)(PPh3)2. Binding modes As ligands, fullerenes behave similarly to electron-deficient alkenes such as tetracyanoethylene. Thus, their complexes are a subset of metal-alkene complexes. They almost always coordinate in a Hapticity, dihapto fashion and prefer electron-rich metal centers.Spessard, p. 162 This binding occurs on the junction of two 6-membered rings. Hexahapto and pentahapto bonding is rarely observed.Spessard, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloro(1,5‐cyclooctadiene)palladium
Dichloro(1,5-cyclooctadiene)palladium is the organopalladium compound with the formula PdCl2(C8H12) where C8H12 is cycloocta-1,5-diene (cod) or abbreviated PdCl2(cod). It is a yellow solid that is soluble in chloroform. According to X-ray crystallography, the Pd center is square planar. This complex can be synthesized by reaction of tetrachloropalladate in hydrochloric acid with cycloocta-1,5-diene. See also *Dichloro(cycloocta-1,5-diene)platinum(II) Dichloro(1,5-cyclooctadiene)platinum(II) (Pt(cod)Cl2) is an organometallic compound of platinum. This colourless solid is an entry point to other platinum compounds through the displacement of the cod and/or chloride ligands. It is one of several ... References Palladium compounds Homogeneous catalysis Chloro complexes Cyclooctadiene complexes {{Chem-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PdCl2(cod)sample
Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures. Structure Two forms of PdCl2 are known, denoted α and β. In both forms, the palladium centres adopt a square-planar coordination geometry that is characteristic of Pd(II). Furthermore, in both forms, the Pd(II) centers are linked by μ2-chloride bridges. The α-form of PdCl2 is a polymer, consisting of "infinite" slabs or chains. The β-form of PdCl2 is molecular, consisting of an octahedral cluster of six Pd atoms. Each of the twelve edges of this octahedron is spanned by Cl−. PtCl2 adopts similar structures, whereas NiCl2 adopts the CdCl2 motif, featuring hexacoordinated Ni(II). Two further polymorphs, γ-PdCl2 and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sonogashira Coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide. :* : aryl or vinyl :* R2: arbitrary :* X: I, Br, Cl or OTf The Sonogashira cross-coupling reaction has been employed in a wide variety of areas, due to its usefulness in the formation of carbon–carbon bonds. The reaction can be carried out under mild conditions, such as at room temperature, in aqueous media, and with a mild base, which has allowed for the use of the Sonogashira cross-coupling reaction in the synthesis of complex molecules. Its applications include pharmaceuticals, natural products, organic materials, and nanomaterials. Specific examples include its use in the synthesis of tazarotene, which is a treatment for psoriasis and acne, and in the preparation of SIB-1508Y, also known as Altinicline, a nicotinic rece ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |