|
Cinpanemab
Cinpanemab (, ; developmental code names BIIB054, NI-202) is an anti-α-synuclein drug acting as a monoclonal antibody against α-synuclein which was under development for the treatment of Parkinson's disease. It showed no significant influence on disease progression in a 52-week phase 2 clinical trial. The drug reached phase 2 clinical trials but was discontinued in 2021 due its lack of effectiveness. It was under development by Biogen. See also * Prasinezumab Prasinezumab (, ; developmental code names NEOD002, PRX-002, RG-7935, RO-7046015) is an anti-α-synuclein drug acting as a monoclonal antibody against α-synuclein which is under development for the treatment of Parkinson's disease. No signifi ... References External links Cinpanemab - AlzForum {{Monoclonals for bone, musculoskeletal, circulatory, and neurologic systems Experimental drugs Experimental monoclonal antibodies Parkinson's disease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prasinezumab
Prasinezumab (, ; developmental code names NEOD002, PRX-002, RG-7935, RO-7046015) is an anti-α-synuclein drug acting as a monoclonal antibody against α-synuclein which is under development for the treatment of Parkinson's disease. No significant effect on disease progression was seen in a 52-week phase 2 clinical trial.There have been concerns about research misconduct and data fabrication relevant to prasinezumab. As of May 2024, prasinezumab is in phase 3 clinical trials for Parkinson's disease. It is under development by Prothena Biosciences and Roche. Mechanism of action Prasinezumab is a humanized IgG1 monoclonal antibody that selectively binds to aggregated forms of alpha-synuclein while sparing the physiological monomeric form. The antibody recognizes the C-terminus of α-synuclein and preferentially targets pathological aggregates that form insoluble fibrils and Lewy bodies—hallmark features of Parkinson's disease pathology. Development history Prasinezumab was or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
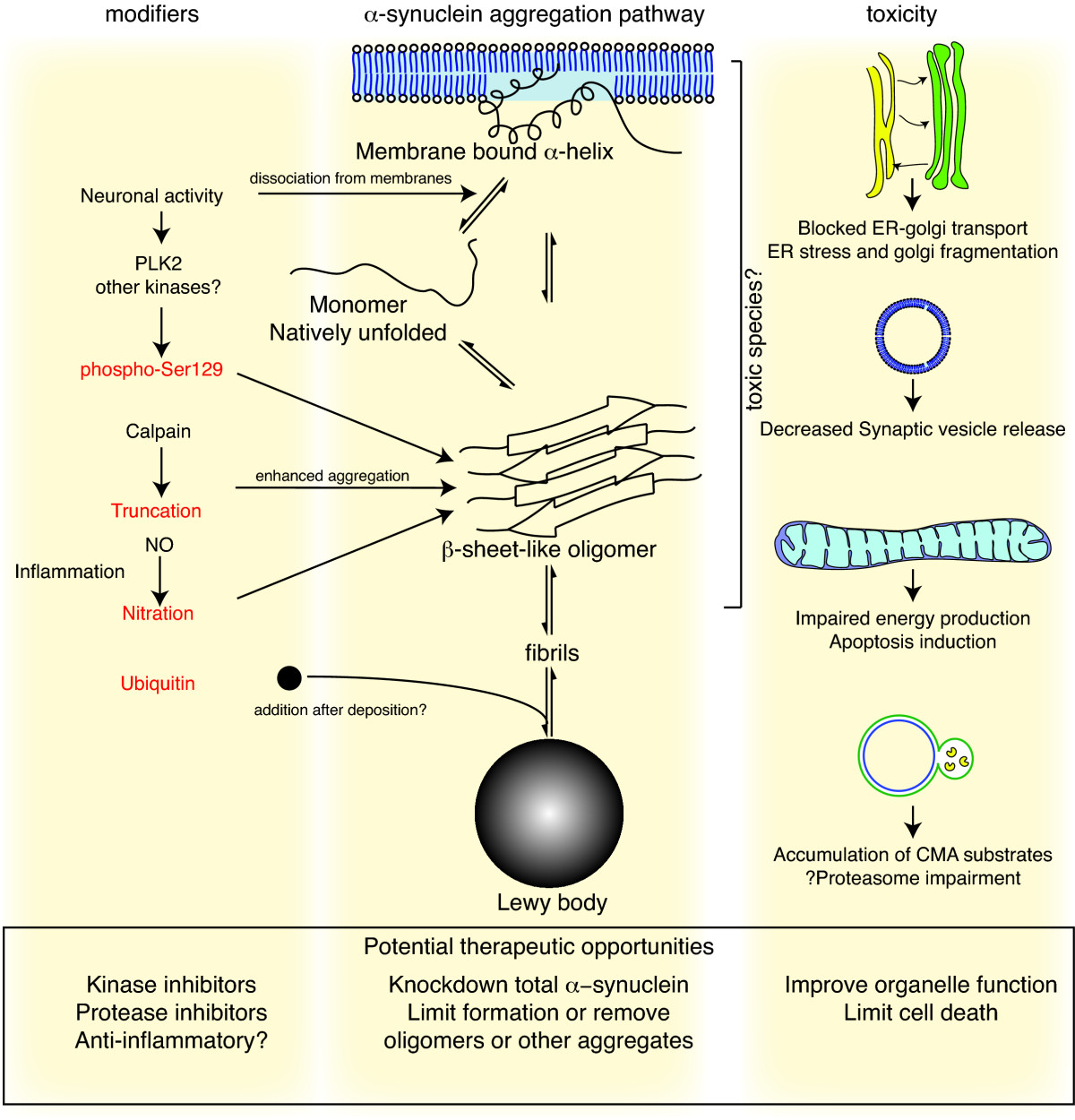
α-Synuclein
Alpha-synuclein (aSyn) is a protein that in humans is encoded by the ''SNCA'' gene. It is a neuronal protein involved in the regulation of synaptic vesicle trafficking and the release of neurotransmitters. Alpha-synuclein is abundant in the brain, with smaller amounts present in the heart, muscles, and other tissues. Within the brain, it is primarily localized to the axon terminals of presynaptic neurons. There, it interacts with phospholipids and other proteins. Presynaptic terminals release neurotransmitters from specialized compartments called synaptic vesicles, a process essential for neuronal communication and normal brain function. In Parkinson's disease and related synucleinopathies, abnormal, insoluble forms of alpha-synuclein accumulate within neurons as inclusions known as Lewy bodies. Mutations in the ''SNCA'' gene are linked to familial forms of Parkinson's disease. During the process of seeded nucleation, alpha-synuclein adopts a cross-beta sheet structure charac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-α-synuclein Drug
An anti-α-synuclein drug, or an α-synuclein inhibitor, is a drug which blocks or inhibits α-synuclein. α-Synuclein is a protein which is thought to be involved in the development and progression of α-synucleinopathies including Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Anti-α-synuclein drugs are under development for treatment of Parkinson's disease and other α-synuclein-related diseases. Examples include the monoclonal antibodies prasinezumab and cinpanemab, which both failed to show effectiveness in slowing the progression of Parkinson's disease in phase 2 clinical trials. Other anti-α-synuclein drugs, like the monoclonal antibody exidavnemab, the α-synuclein vaccines PD01A and PD03A, and the small-molecule α-synuclein misfolding and aggregation inhibitors minzasolmin and emrusolmin, are also under development. Memantine is also being studied as a potential disease-modifying treatment for Parkinson's disease by inhibiting cell-t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibody
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies are identical and can thus have monovalent affinity, binding only to a particular epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies are mixtures of antibodies derived from multiple plasma cell lineages which each bind to their particular target epitope. Artificial antibodies known as bispecific monoclonal antibodies can also be engineered which include two different antigen binding sites ( FABs) on the same antibody. It is possible to produce monoclonal antibodies that specifically bind to almost any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-synuclein
Alpha-synuclein (aSyn) is a protein that in humans is encoded by the ''SNCA'' gene. It is a neuronal protein involved in the regulation of synaptic vesicle trafficking and the release of neurotransmitters. Alpha-synuclein is abundant in the brain, with smaller amounts present in the heart, muscles, and other tissues. Within the brain, it is primarily localized to the axon terminals of presynaptic neurons. There, it interacts with phospholipids and other proteins. Presynaptic terminals release neurotransmitters from specialized compartments called synaptic vesicles, a process essential for neuronal communication and normal brain function. In Parkinson's disease and related synucleinopathies, abnormal, insoluble forms of alpha-synuclein accumulate within neurons as inclusions known as Lewy bodies. Mutations in the ''SNCA'' gene are linked to familial forms of Parkinson's disease. During the process of seeded nucleation, alpha-synuclein adopts a cross-beta sheet structure charac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parkinson's Disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor system, motor and non-motor systems. Symptoms typically develop gradually and non-motor issues become more prevalent as the disease progresses. The motor symptoms are collectively called parkinsonism and include tremors, bradykinesia, spasticity, rigidity as well as postural instability (i.e., difficulty maintaining balance). Non-motor symptoms develop later in the disease and include behavior change (individual), behavioral changes or mental disorder, neuropsychiatric problems such as sleep abnormalities, psychosis, anosmia, and mood swings. Most Parkinson's disease cases are idiopathic disease, idiopathic, though contributing factors have been identified. Pathophysiology involves progressive nerve cell death, degeneration of nerve cells in the substantia nigra, a midbrain region that provides dopamine to the basal ganglia, a system invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Description Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. When expressed specifically, a clinical trial phase is capitalized both in name and Roman numeral, such as "Phase I" clinical trial. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory aut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biogen
Biogen Inc. is an American multinational biotechnology company based in Cambridge, Massachusetts, United States specializing in the discovery, development, and delivery of the treatment of neurological diseases to patients worldwide. Biogen operates in Argentina, Brazil, Canada, China, France, Germany, Hungary, India, Italy, Japan, Mexico, Netherlands, Poland, Sweden, and Switzerland. History Biogen was founded in 1978 in Geneva as ''Biotechnology Geneva'' by several prominent biologists, including Kenneth Murray from the University of Edinburgh, Phillip Allen Sharp from the Massachusetts Institute of Technology, Walter Gilbert from Harvard University (Gilbert served as CEO during the start-up phase of Biogen), Heinz Schaller from the University of Heidelberg, and Charles Weissmann from the University of Zurich (Weissmann contributed the first product interferon alpha).Werner Grundlehner''Zürcher Antikörper gegen Alzheimer hat Milliardenpotenzial – und Gegenwind.''Neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Experimental Drugs
An experiment is a procedure carried out to support or refute a hypothesis, or determine the efficacy or likelihood of something previously untried. Experiments provide insight into cause-and-effect by demonstrating what outcome occurs when a particular factor is manipulated. Experiments vary greatly in goal and scale but always rely on repeatable procedure and logical analysis of the results. There also exist natural experimental studies. A child may carry out basic experiments to understand how things fall to the ground, while teams of scientists may take years of systematic investigation to advance their understanding of a phenomenon. Experiments and other types of hands-on activities are very important to student learning in the science classroom. Experiments can raise test scores and help a student become more engaged and interested in the material they are learning, especially when used over time. Experiments can vary from personal and informal natural comparisons ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |