|
Chemical Garden
file:Chemical garden.jpg, Comparison of chemical gardens grown by NASA scientists on the International Space Station (left) and on the ground (right) file:Silicate garden growing 0001.ogv, A chemical garden while growing file:Silikatna bašta - kobalt - 002.jpg, upCobalt(II) chloride file:Silikatna bašta 2.JPG, upA chemical garden A chemical garden is a set of complex biological-looking structures created by mixing inorganic chemicals. This experiment in chemistry is usually performed by adding metal salts, such as copper sulfate or cobalt(II) chloride, to an aqueous solution of sodium silicate (otherwise known as waterglass). This results in the growth of plant-like forms in minutes to hours. The chemical garden was first observed and described by Johann Rudolf Glauber in 1646. In its original form, the chemical garden involved the introduction of ferrous chloride (FeCl2) crystals into a solution of potassium silicate (K2SiO3). Process The chemical garden relies on most tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Displacement Reaction
A salt metathesis reaction (also called a double displacement reaction, double replacement reaction, or double decomposition) is a type of chemical reaction in which two ionic compounds in aqueous solution exchange their component ions to form two new compounds. Often, one of these new compounds is a precipitate, gas, or weak electrolyte, driving the reaction forward. :AB + CD -> AD + CB \mathitA_\mathitD_\mathit + \mathitC_\mathitB_\mathit --> In older literature, the term double decomposition is common. The term double decomposition is more specifically used when at least one of the substances does not dissolve in the solvent, as the ligand or ion exchange takes place in the solid state of the reactant. For example: :AX(aq) + BY(s) → AY(aq) + BX(s). Types of reactions Counterion exchange Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guided by a solubility chart or lattice energy. HSAB theory can also be used to predict ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Portland Cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in the early 19th century by Joseph Aspdin, and is usually made from limestone. It is a fine powder (substance), powder, produced by heating limestone and clay minerals in a kiln to form Clinker (cement), clinker, and then #Cement grinding, grinding the clinker with the addition of several percent (often around 5%) gypsum. Several types of portland cement are available. The most common, historically called ordinary portland cement (OPC), is grey, but white portland cement is also available. The cement was so named by Joseph Aspdin, who obtained a patent for it in 1824, because, once hardened, it resembled the fine, pale limestone known as Portland stone, quarried from the windswept cliffs of the Isle of Portland in Dorset. Portland stone was p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Sulfate
Zinc sulfate is an inorganic compound with the formula ZnSO4. It forms hydrates ZnSO4·''n''H2O, where ''n'' can range from 0 to 7. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the chemical formula, formula . As early as the 16th century it was prepared on a large scale, and was historically known as "white vitriol" (the name was used, for example, in 1620s by the collective writing under the pseudonym of Basil Valentine). Zinc sulfate and its hydrates are colourless solids. Uses Manufacturing The main application of the heptahydrate is as a coagulant in the production of rayon. It is also a precursor to the pigment lithopone. It is also used as an electrolyte for zinc electroplating, as a mordant in dyeing, and as a preservative for skins and leather. Nutrition Zinc sulfate is used to supply zinc in animal feeds, fertilizers, toothpaste, and agricultural sprays. Zinc sulfate, like many zinc compounds, can be used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Chloride
Calcium chloride is an inorganic compound, a Salt (chemistry), salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide. Calcium chloride is commonly encountered as a Water of crystallization, hydrated solid with generic formula , where ''n'' = 0, 1, 2, 4, and 6. These compounds are mainly used for de-icing and dust control. Because the anhydrous salt is Hygroscopic, hygroscopic and deliquescent, it is used as a desiccant.Robert Kemp, Suzanne E. Keegan "Calcium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. History Calcium chloride was apparently discovered in the 15th century but wasn't studied properly until the 18th century. It was historically called "fixed Salammoniac, sal ammoniac" () because it was synthesized during the distillation of ammonium chloride with lime and was nonvolatile (while ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III) Chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms, which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals. Electronic and optical properties All forms of ferric chloride are paramagnetic, owing to the presence of unpaired electrons residing in 3d orbitals. Although Fe(III) chloride can be octahedral or tetrahedral (or both, see structure section), all of these forms have five unpaired electrons, one per d-orbital. The high spin d5 electronic configuration requires that d-d electronic transitions are spin forbidden, in addition to violating the Laporte rule. This double forbidden-ness results in its solutions being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Sulfate
Iron(II) sulfate or ferrous sulfate (British English: sulphate instead of sulfate) denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the heptahydrate (''x'' = 7), but several values for x are known. The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol ( vitriol is an archaic name for hydrated sulfate minerals), the blue-green heptahydrate ( hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex e(H2O)6sup>2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas. It is on the World Health Organization's List o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Sulfate
Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble turquoise coloured salt is a common source of the Ni2+ ion for electroplating. Approximately 40,000 tonnes were produced in 2005.K. Lascelles, L. G. Morgan, D. Nicholls, D. Beyersmann “Nickel Compounds” in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Vol. A17 p. 235 . Structures At least seven sulfate salts of nickel(II) are known. These salts differ in terms of their hydration or crystal habit. The common tetragonal hexahydrate crystallizes from aqueous solution between 30.7 and 53.8 °C. Below these temperatures, a heptahydrate crystallises, and above these temperatures an orthorhombic hexahydrate forms. The yellow anhydrous form, NiSO4, crystallizes in orthorhombic crystal system and in standard pressure decomposes to NiO in temperatures above 640 °C, before reaching the melting point. It melts only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(III) Chloride
Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula . This crystalline salt forms several hydrates with the formula , among which are hydrates where ''n'' can be 5 (chromium(III) chloride pentahydrate ) or 6 (chromium(III) chloride hexahydrate ). The anhydrous compound with the formula are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, . Chromium chlorides find use as catalysts and as precursors to dyes for wool. Structure Anhydrous chromium(III) chloride adopts the structure, with occupying one third of the octahedral interstices in alternating layers of a pseudo- cubic close packed lattice of ions. The absence of cations in alternate layers leads to weak bonding between adjacent layers. For this reason, crystals of cleave easily along the planes between layers, which results in the flaky (micaceous) appearance of samples of chromium(III) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Sulfate
Copper(II) sulfate is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper,Antoine-François de Fourcroy, tr. by Robert Heron (1796) "Elements of Chemistry, and Natural History: To which is Prefixed the Philosophy of Chemistry". J. Murray and others, Edinburgh. Page 348. and Roman vitriol.Oxford University Press,Roman vitriol, Oxford Living Dictionaries. Accessed on 2016-11-13 It exothermically dissolves in water to give the aquo complex , which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The centers are interconnected by sulfate anions to form chains. Preparation and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , such that is a valence (chemistry), monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with the formula . Other alums are named after the monovalent ion, such as sodium alum and ammonium alum. The name "alum" is also used, more generally, for salts with the same formula and structure, except that aluminium is replaced by another Valence (chemistry), trivalent metal ion like chromium#Chromium(III), chromium, or sulfur is replaced by another chalcogen like selenium. The most common of these analogs is chrome alum . In most industries, the name "alum" (or "papermaker's alum") is used to refer to aluminium sulfate, , which is used for most industrial flocculation (the variable is an integer whose size depends on the amount of water absorbed into the alum). For medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
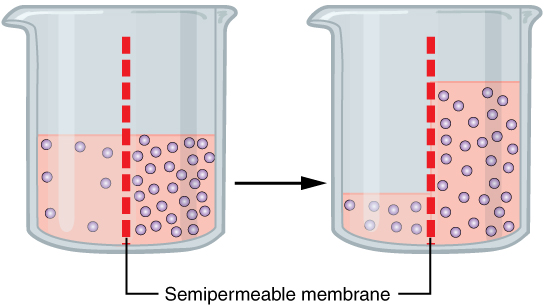
Osmotic
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to prevent net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semipermeable. In general, thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |